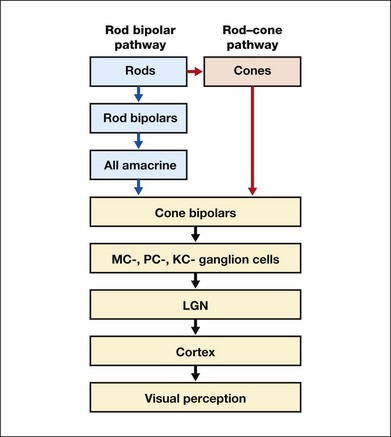
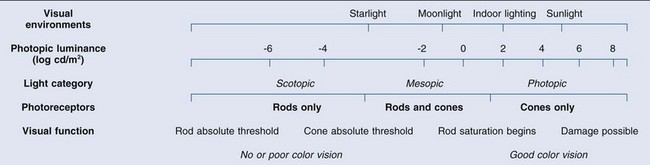
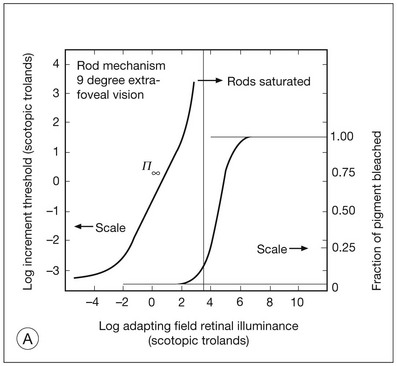
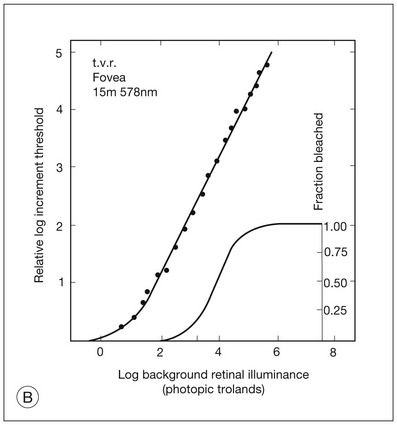
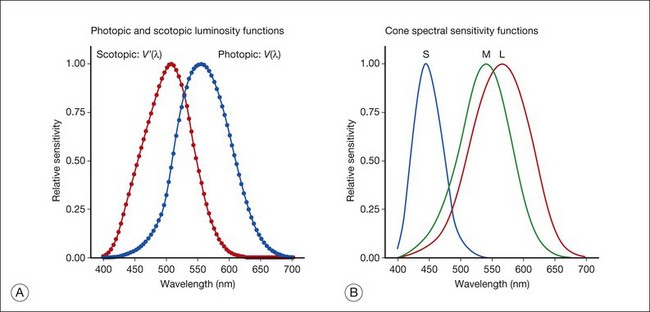
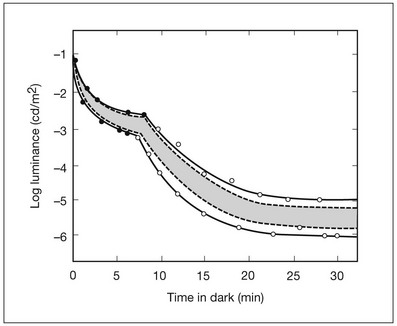
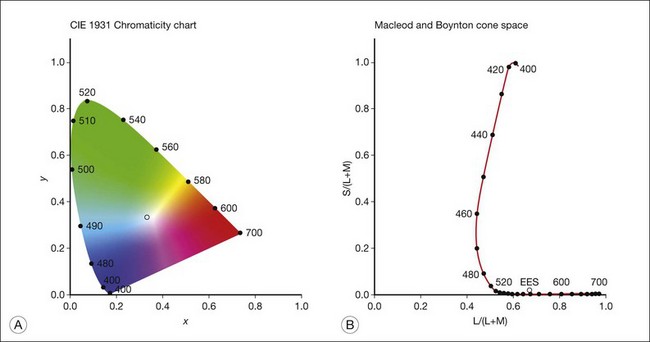
Chapter 10 Differences in the anatomy and physiology (see Chapters 4, Autofluorescence imaging, and 9, Diagnostic ophthalmic ultrasound) of the rod and cone systems underlie different visual functions and modes of visual perception. The rod photoreceptors are responsible for our exquisite sensitivity to light, operating over a 108 (100 millionfold) range of illumination from near total darkness to daylight. Cones operate over a 1011 range of illumination, from moonlit night light levels to light levels that are so high they bleach virtually all photopigments in the cones. Together the rods and cones function over a 1014 range of illumination. Depending on the relative activity of rods and cones, a light level can be characterized as photopic (cones alone mediate vision), mesopic (both rods and cones are active), or scotopic (rods alone mediate vision).1 In the literature, the terms photopic vision and scotopic vision are used to reflect cone and rod vision, respectively. Table 10.1 shows this overlapping range of activity. Table 10.1 The dynamic range of the human visual system Modified from Hood DC, Finkelstein MA. Sensitivity to light. In: Boff KR, Kaufman L, Thomas JP, editors. The handbook of perception and human performance, vol. 1. Sensory processes and perception. New York: John Wiley; 1986. The distribution of rods and cones in the retina (see Chapter 4, Autofluorescence imaging) is also reflected in visual function. The greatest sensitivity to light occurs in the midperiphery of the visual field, which has a predominance of rods, while high-acuity and good color vision are mediated by the fovea, which has a predominance of cones. Nonetheless, the entire retina, with the exception of a very small area within the fovea, is capable of mediating night vision, and color vision is present throughout the visual field with daylight stimulation of the entire retina. The following will introduce rod and cone differences in light adaptation, spectral sensitivity, and spatial/temporal sensitivity. Photoreceptors, whether they are rods or cones, respond well to only a small range of variations in illumination within a steady adapting background.2 However, adaptation mechanisms adjust photoreceptor sensitivity so that this small range of responses is always centered near the current adaptation level, even though adaptation levels can vary over a wide range. This behavior forms the basis for the large operating range of the visual system. It is possible to measure a threshold for the perception of an increment in light on a large, steady background field. As the background light level is increased, the increment threshold starts to increase. Rods and cones behave somewhat differently in this regard. For the rod system, as shown in Fig. 10.1A, the increment threshold increases steadily over almost a thousand-fold range. With further increases in background adaptation levels, an increment is not detected, no matter how much additional test light is presented as an increment, due to rod saturation. In comparison, the cone system, as shown in Fig. 10.1B, shows a continuous steady increase in the increment threshold with increases in background illumination, even at levels that bleach almost the entire amount of available photopigment. The portion of the curve that rises linearly with illumination levels is called the Weber region (Fig. 10.1). In the Weber region, an incremental light can be detected when it is a constant proportion (i.e., the Weber fraction) of the background light level. Different photoreceptor systems have a characteristic Weber fraction. Cones have lower Weber fractions than rods and the M and L cones have lower Weber fractions than S cones. Under optimal conditions, the cone system can detect a light level difference of 1%, while rods need a light change of 20%. Fig. 10.1 The increment threshold functions for scotopic (rod) and photopic (cone) vision as a function of background illuminance. (A) Rod increment thresholds measured at 9° in the parafovea. The dashed line has a slope of 1. The portion where the curve has unit slope (in parallel to the dashed line) is the Weber region, followed at higher levels by the region of rod saturation. To the right is shown the fraction of rod photopigment bleached. The rods are saturated before there is substantial photopigment bleaching. (B) Cone increment thresholds measured at the fovea. To the right is shown the fraction of photopigment bleached. The Weber region extends to luminances (6 million tds) that bleach virtually all the cone photopigment. (Reproduced with permission from Enoch JM. The two-color threshold technique of Stiles and derived component color mechanisms. In: Jameson D, Hurvich L, editors. Visual psychophysics: handbook of sensory physiology, vol. VII/4. Berlin: Springer-Verlag; 1972.) In addition to photoreceptor adaptational properties, other factors, including pupil size, the temporal and spatial summation characteristics, and photopigment depletion, can also contribute to extend the operating range of the visual system over a large luminance range. While some adaptation operates within the photoreceptors themselves, other properties of adaptation may reflect the effects of the complex neural circuitry of the retina.2 Day vision is primarily mediated by three types of cone photoreceptors with different but overlapping spectral sensitivities. Each is identified by the relative position of the peak in spectral sensitivity. The three cone types are called the long-, middle-, and short-wavelength-sensitive (L, M, and S) cones. When overall sensitivity to light is measured at the light-adapted fovea, a broad sensitivity spectrum peaking near 555 nm is found. This sensitivity spectrum represents the combined activity of the L and M cones and is called the V(λ) function. When sensitivity to light is measured in the dark-adapted peripheral retina, where rods dominate, a broad-sensitivity spectrum is found with a peak sensitivity at 507 nm. This rod spectral sensitivity function is called V’(λ) (Fig. 10.2A). Both V(λ) and V’(λ) functions have practical significance and have been accepted by the International Commission of Illumination as representative of human vision relative luminous efficiency at photopic and scotopic levels. They are also used to relate luminous (perceived energy of light) to radiant (emitted light) energy. Fig. 10.2 Spectral sensitivities of cones and rods. (A) The relative spectral luminous efficiency functions for scotopic and photopic vision adopted by the Commission International d’Eclairage (CIE), V ’(λ) and V(λ) respectively. (Data from Wyszecki G, Stiles WS. Color science – concepts and methods, quantitative data, and formulae, 2nd edn. New York: John Wiley; 1982). (B) Spectral sensitivities of the S, M, and L cones derived from color-matching function.14 Estimates of the spectral sensitivities of the three cone types have been obtained from a variety of psychophysical procedures. One approach was to derive the spectral sensitivities of cones from analysis of color-matching data. Another approach used spectral bleaching lights to depress the responses of two cone types relative to the third so that measurements of the spectral sensitivity of the third cone type could be isolated. Figure 10.2B shows the relative spectral sensitivities of the three cone types. The S cones are most sensitive to light near 445 nm, with sensitivity declining rapidly at longer wavelengths. At 555 nm and longer wavelengths, the S cones are virtually unresponsive to light. The M and L cones have overlapping spectral sensitivities that span the entire visible spectrum. The M cones peak in sensitivity near 543 nm, while the L cones peak near 566 nm. The differential spectral sensitivity functions of the L, M, and S cones provide the foundation of early spectral processing. Compared with the cone system, the rod system has poorer spatial resolution (acuity). For an observer with 20/20 photopic acuity, scotopic acuity would be about 20/200 (10 times worse than photopic acuity). The rod system also has poorer temporal resolution, which refers to the ability to perceive a physically alternating light as steady or flickering in time. The transitional temporal frequency at which the light appears from flickering to steady is called the critical fusion frequency (CFF). CFFs increase with light adaptation level, reaching a maximum of 20 Hz for rods and 55–60 Hz for cones. This means that flickering lights can be perceived at higher frequencies in brighter light conditions. Interestingly, dark-adapted rods can suppress cone-mediated flicker detection3 and this suppression mainly occurs in the magnocellular (MC) pathway (explained below).4 Rod signals are conveyed by two primary neural pathways that are dependent on the illumination level.5 One pathway is via ON rod bipolars, AII amacrine cells, and ON and OFF cone bipolars, which are all cells in the retina. This is a temporally sluggish pathway that mediates rod vision at low scotopic light levels. The second pathway transmits rod information via rod–cone gap junctions and ON and OFF cone bipolar cells in the retina. This is a fast pathway that mediates vision at higher scotopic and mesopic light levels. A third insensitive rod pathway between rods and OFF cone bipolars has been identified in rodents but, thus far, not in primates. The significant point here is that rods and cones share neural pathways and have joint inputs to retinal ganglion cells. There are three major neural retinogeniculate pathways in primates that convey retinal information to the visual cortex.6,7 The pathways are named after the layers of the lateral geniculate nucleus (LGN) that receive input from distinct types of ganglion cells and project to different areas of the primary visual cortex. The MC layer of the LGN receives inputs from parasol ganglion cells. The MC pathway processes the summed output of the L and M cones to signal luminance information. The parvocellular (PC) layer of the LGN receives input from midget ganglion cells. The PC pathway mediates spectral opponency of L and M cones (discussed later) to signal chromatic information. The koniocellular (KC) layer of the LGN, which receives input from small bistratified as well as other ganglion cells, detects changes in S-cone signals compared to the sum of the L- and M-cone signals. These three pathways mediate different aspects of vision, with the MC pathway mainly carrying out luminance and motion processing, the PC pathway mainly processing red–green color, acuity, and shape information, and the KC pathway mainly handling blue–yellow color processing. The sharing of neural pathways between rods and cones implies that rods should have input to the MC, PC, and KC pathways. Indeed, physiological studies have shown that there is strong rod input to the MC pathway, but weak input to the PC pathway.8 Demonstration of rod input to the KC pathways is less clear. An earlier study8 did not find rod input to the bistratified ganglion cells in the parafovea, while two recent studies demonstrated strong rod input in the peripheral retina.9,10 Figure 10.3 shows a schematic diagram of the visual pathways conveying rod and cone inputs to the MC, PC, and KC ganglion cells, which would then produce signals that are projected into the cortex to mediate different aspects of visual perception. Measurements of sensitivity thresholds during adaptation to darkness have produced a characteristic biphasic function with an initial segment that is attributed to cone responses and a subsequent segment attributed to rod responses. Figure 10.4 shows a characteristic dark adaptation function measured in the peripheral retina. Thresholds decrease quickly initially and this rapid recovery is attributed to cones. Thresholds then reach a plateau in about 5 minutes and remain invariant for another 5 minutes (cone plateau, reflecting cone absolute thresholds). Then, there is a second rapid decrease in thresholds due to sensitivity recovery of the rods, referred to as the rod–cone break, to a new plateau that is reached in 40–50 minutes (rod plateau, reflecting rod absolute thresholds). Fig. 10.4 The time course of dark adaptation. The range of threshold sensitivities of 110 normal observers is shown. The circular points represent data of the most and least sensitive individuals. The area enclosed by the dashed lines represents 80% of this population. The cone absolute thresolds are obtained during the cone and rod plateaus, respectively. (Data from Hecht S, Mandlebaum J. The relationship between vitamin A and dark adaptation. JAMA 1939;112:1910–6.) The shape of the dark adaptation function depends on testing parameters, including retinal location, wavelength, and temporal and spatial characteristics.1,11 The effects of these parameters on dark adaptation curves can be understood by the differences between rods and cones in terms of their distributions, spectral sensitivity, and spatial/temporal resolution characteristics. For instance, because there are only cones in the fovea, dark adaptation measured at the fovea using a small test light reveals a rapid monophasic branch attributable to the cones. On the other hand, because the rod and cone systems have similar absolute sensitivities at long wavelengths, dark adaptation measured with long-wavelength lights is monophasic, resembling the cone function. As the test wavelength is changed to shorter wavelengths, a biphasic curve emerges because the rods show greater absolute sensitivity than cones at shorter wavelengths.12 It is known that certain retinal disorders may selectively affect rods (e.g., retinitis pigmentosa) or cones (e.g., cone dystrophies). Clinically, rod and cone functions can be evaluated electrophysiologically by measuring rod and cone electroretinograms (ERG: see Chapter 7, Electrogenesis of the electroretinogram) or, psychophysically, by measuring dark adaptation functions. Dark adaptation functions quantify the ability of the rod and cone systems to recover sensitivity (i.e., regenerate photopigment) after exposure to light. The recovery is faster for cones, but the absolute level of sensitivity is greatest for rods. Variations in sensitivity and sensitivity recovery times can be used to characterize retinal disorders. Color vision refers to our ability to perceive colors based on spectral variations in light absorbed by the photoreceptors. Color vision includes both chromatic discrimination and color appearance appreciation. Color matching and color discrimination experimental tasks are two fundamental psychophysical procedures that have provided theoretical insights into the nature of color vision and have also been developed for clinical diagnosis of color vision. Color matching and color discrimination results, however, do not address questions of color appearance, for example, why an object appears red. Color appearance is far more complex because it depends on not only the chromatic properties of an object but also the spatial, temporal, and spectral characteristics of the neighboring objects.13 Neural processing beyond the retina is required for color appearance perceptions. Color matching as the foundation for the theory of trichromacy The psychophysical procedure in which an observer sets a mixture of three primary lights to match the color of a test stimulus is called color matching. It has been known since the 19th century that different colors perceived by humans can be specified by an economical three-variable (trichromatic) system. In the 1800s, Thomas Young and Hermann von Helmholtz proposed that there must be three kinds of physiological entities in the eye accounting for this trichromacy and their theory is called the Young–Helmholtz trichromatic theory of color vision. We now know the basis for trichromacy is the existence of three cone types in the retina. Color matching was the means to uncover the spectral sensitivity functions of the three different cone types (Fig. 10.1B).14 Figure 10.5A shows the 1931 CIE chromaticity chart, in which the coordinates of the Y primary [y = Y/(X + Y + Z)] are plotted against those of the X primary [x = X/(X + Y + Z)]. The spectrum loci (their wavelengths are indicated on the graph) form the horseshoe-shaped curve. Equal-energy-spectrum (EES) light is plotted in the center, with the coordinates of x = 0.3333 and y = 0.3333. A straight line connects 400 nm to 700 nm for purples, which result from mixtures of short- and long-wavelength light. All possible lights occur within the boundaries of the spectrum locus and the purple line. Highly saturated colors occur near the locus and desaturated (pale) colors occur near the white point. Fig. 10.5 Chromaticity spaces. (A) The Commission International d’Eclairage (CIE) 1931 x, y chromaticity diagram. An equal-energy-spectrum (EES) light has a coordinate of x = 0.3333 and y = 0.3333. Spectral wavelengths are represented on the horseshoe-shaped spectrum locus. All lights can be represented in this diagram. (Data from Wyszecki G, Stiles WS. Color science – concepts and methods, quantitative data, and formulae, 2nd edn. New York: John Wiley; 1982.) (B) The MacLeod and Boynton cone space, which plots S-cone excitation versus relative L/M-cone excitations. In this space, the S/(L + M) chromaticity of 400 nm spectral light is normalized to be 1. In such a normalization, an EES light has a chromaticity of L/(L + M) = 0.665 and S/(L + M) = 0.016. In a relative cone troland space, the S/(L + M) for an EES light is normalized to be 1. The chromaticities of the spectral loci form an “L” shape in this space.
Color Vision and Night Vision
Rod and cone functions
Light adaptation
Spectral sensitivity
Spatial and temporal resolution
Visual pathways for rod and cone functions
Retinogeniculate pathways
Dark adaptation functions: assessment of the shift from day vision to night vision
Clinical evaluation using dark adaptation functions
Color vision
Color matching
The CIE colorimetric system
Color Vision and Night Vision