, Valery Kornienko2 and Igor Pronin2
(1)
N.N. Blockhin Russian Cancer Research Center, Moscow, Russia
(2)
N.N. Burdenko National Scientific and Practical Center for Neurosurgery, Moscow, Russia
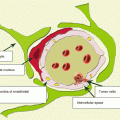
Colorectal cancer (CRC) is a general term determining the concepts of colon cancer and rectal cancer. It combines malignant epithelial tumors of the cecum, colon, rectum, and anal canal; varies by their form, location, and histological structure; and constitutes 15% of all primary tumors diagnosed.
According to the World Health Organization, more than 900,000 new cases of colorectal cancer (CRC) are diagnosed in the world each year. The highest incidence is observed in the USA, Canada, Western Europe, and Russia. An increase in the incidence by 14.7% in men and 18.0% in women was noted from 3 to 1997 in Russia (Martyniuk 2000). Subsequent studies showed that this trend continues from 2003 to 2008, and the incidence increased by 11.6% in men and 13.4% women (Davydov and Axel 2010). According to forecasts, in Russia in years to come, CRC will be leading among tumors of the gastrointestinal tract, as is currently the case in most developed countries.
Mortality from colorectal cancer remains high. The largest mortality from CRC is reported in the Czech Republic, Hungary (34.3 per 100,000 people), and New Zealand (26.4), while the lowest (15.2) is in the USA (Khanevich et al. 2008). In Russia, CRC occupies the third place in terms of mortality from all malignant tumors. In Western Europe, due to active screening programs, about 80% of patients have tumors that can be surgically removed (Vogel et al. 2000). The five-year survival in colorectal cancer is about 60% in developed countries and less than 40% in resource-limited countries.
A major factor in the development of colorectal cancer is advanced age: the likelihood of CRC substantially increases after the age of 55 years and becomes the highest after 70–75 years of age (Boyle and Leon 2002; Faivre et al. 2002; Papapolychroniadis 2004), although patients in 7% of cases are younger than 50 years old. According to various sources, the hereditary factor plays a role in 6–30% of cases: familial adenomatous polyposis or hereditary nonpolyposis colorectal cancer (Lynch syndrome). Among other factors associated with an increased risk of colorectal cancer, there are chronic inflammatory diseases of the colon—ulcerative colitis and Crohn’s disease with colon involvement. However, according to Burt et al. (1990), in 75%, CRC occurs without any known predisposing factors.
Colorectal cancer most often metastasizes to the lungs (10–20%) and liver (20–30%), less often to the spine, uterus, adrenal glands, pancreas, and brain (Patanaphan and Salaazar 1993; Tan et al. 2009). According to Graf et al. (1988), CRC metastases comprise 1.8–4.8% of all metastatic brain lesions. According to other authors, the incidence of secondary brain involvement reaches 10% in patients with colorectal cancer (Temple et al. 1982). The median interval from CRC diagnosis to detection of metastases in the brain is 22–33 months. In our sample, patients with colorectal cancer metastases in the brain made up 5.4% of all metastatic lesions of this type of cancer.
The probability of brain metastases (including micrometastases) cannot be ruled out in patients with localized forms of gastrointestinal cancer. Thus, according to Vogel et al. (2000), tumor cells circulating in the blood were found in 40%, while micrometastases in the bone marrow were detected in 39% of patients with stage I–II colon cancer. This substantiates the advisability of adjuvant drug therapy after a curative surgery for colorectal cancer in order to improve long-term results of the treatment.
A native (without contrast enhancement) CT study of colorectal cancer metastases visualizes rounded lesions with isodense or slightly decreased density. It is often difficult to establish a clear boundary between the tumor edge and a perifocal edema. In small tumor lesions, it can be identified by a possible presence of indirect signs—edema of the brain substance, accompanying the tumor. The accumulation of the contrast agent by the metastasis helps clearly visualize its external borders and allows to differentiate it from the brain tissue edema; CRC metastases accumulate radiopaque contrast agents well.
Despite pronounced CA accumulation by the solid part of colorectal cancer metastases , CBV values in such cases appeared to be significantly lower and quantitatively similar to CBV of those for metastases of breast cancer and uterine and lung cancer (CBV = 8.14 ± 3.71 (ml/100 g)). Average CBF values in colorectal cancer metastases (as well as in renal cancer metastases) are low and quantitatively similar, CBF = 72.13 ± 35.35 (ml/100 g/min), but with a large dispersion of individual values. MTT values obtained (7.72 ± 3.26 s) were not specific for CRC as compared to MTT values in metastatic tumors with another morphogenesis.
As noted, the use of CT perfusion imaging in follow-up studies after radiosurgery performed for colorectal cancer metastases in the brain may be useful not only in terms of assessing the effectiveness of the treatment but also, in some of the cases, allowing to determine the presence of continued growth of the metastatic lesion, not detectable with MRI.
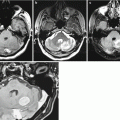
MRI in colorectal cancer is the preferred alternative to CT. The contrast agent accumulation in MRI in colorectal cancer metastases is pronounced, with fairly clear, sometimes bumpy contours; however, the lesion borders are always well determined. An MR picture on T1-weighted MRI + Gd of colorectal cancer metastases in the brain somewhat resembles that in metastases of uterine cancer .
Particularly noteworthy are metastases of colorectal adenocarcinoma, which were characterized by a hypointense signal on T2-weighted MRI in the majority (83%) of cases in our clinical material. Some of them showed signs of moderate central necrosis, characterized by a higher signal on T2-weighted MRI. Perifocal edema in the area of colorectal cancer metastases is quite expressed, which indicates active growth of the tumor. As indicated above, such MR tissue characteristics with an iso-hypointense signal on T2-weighted MRI were obtained in cases of pigmented melanoma metastases in the brain, which was due to the paramagnetic properties of melanin, but no evidence of increased signal on T1-weighted MRI was observed in colorectal cancer metastases. In metastases from other primary tumors, this pattern was not encountered, and, in case of an unknown primary tumor site, it allowed to suspect the source of metastases. It is assumed that changes in MR characteristics of colorectal adenocarcinoma metastases were associated with mucin content, having paramagnetic properties. However, this hypothesis requires further investigation.
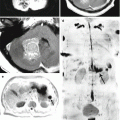
The use of SWI (SWAN) to verify vascular changes in the structure of colorectal cancer metastases in the brain is not informative (as in melanomas), unlike metastases of other histogenesis and primary brain tumors. A characteristic diffuse decrease in MR signal on SWI (SWAN) for CRC metastases in the brain does not allow to use the method as an expert one to evaluate the hemorrhagic component of these tumors. The signal on SWI (SWAN) MRI from CRC metastases, according to our data, was homogeneously reduced in 85% of cases, which distinguishes these metastases from other secondary tumor lesions of the brain.
In the diagnosis of colorectal cancer metastases in the brain, MR spectroscopy identified common findings for metastases of different histogenesis: in the central part of metastases (typically, this is an area of tumor tissue degradation), before the treatment, there is an increase in the peaks of lactate and lipids in all cases; in 10% of cases, there is a moderate choline peak. No MRI spectroscopy findings specific for colorectal cancer metastases in the brains were obtained.
On DWI images, solid components of CRC metastases had an iso-hypointense, homogeneous MR signal; ADC is lower on the border of the metastasis and perifocal edema than in primary brain tumors (p < 0.15).
The complexity of the diagnosis in case of detecting the areas with increased RP accumulation (18F-FDG) in the projection of the intestines is that a PET picture is not specific for tumor lesions, but displays local inflammatory changes, diverticula, erosions, ulcers, and symptoms of diabetes. Therefore, a prerequisite is colonoscopy to obtain a biopsy and a histological examination of the latter. A study of tumor markers (CEA or CA) is also mandatory. When colorectal cancer metastases are large enough, they are well differentiated on the background of intact brain substance.
16.1 Clinical Cases
See Figs. 16.1, 16.2, 16.3, 16.4, 16.5, 16.6, 16.7, 16.8, 16.9, 16.10, 16.11, 16.12, 16.13, 16.14, 16.15, 16.16, 16.17, and 16.18.
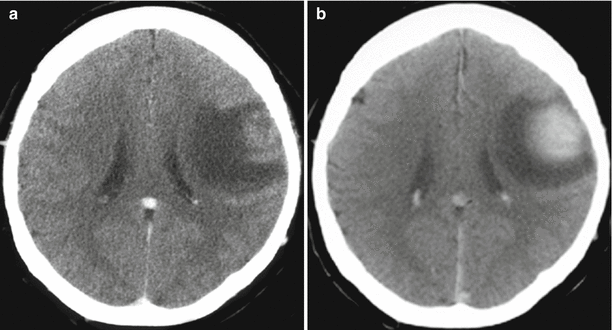

Fig. 16.1




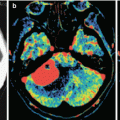
Colorectal cancer metastases in the brain. On a CT scan in the axial projection, there is a large, isodense tumor lesion (a) intensely and homogeneously accumulating the contrast agent on the background of the expressed perifocal edema (b), convexitally, in the left frontal region
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree