- •
Anatomic delineation of complex CHD is just that–complex. No assumptions should be made regarding anatomy; segments (atria, ventricles, and great vessels), intersegmental connections (atria-to-ventricle and ventricle-to-great vessel), and venous structures (systemic and pulmonary) must clearly be defined. The CMR imager needs to be aware of the spectrum of complex CHD along with the myriad of surgical reconstructions that can be performed to accomplish this successfully.
- •
As with anatomy, the physiologic and functional correlates of complex CHD are intricate. Delineation of which way blood is flowing and quantification of flow along with evaluation of ventricular and valve function are crucial to successful medical and surgical management. Understanding complex physiologic and functional principles to sort out the most intricate details is expected, even if the CMR imager has never had the opportunity to study a particular case before.
- •
Spatial relationships, both among the various cardiovascular structures and between cardiovascular structures and other thoracic and abdominal organs, are important to define. This not only aids in diagnosis but also helps the cardiovascular surgeon and pediatric cardiologist plan out repairs. As such, the CMR imager should have a good grasp of how to reconstruct the heart and other organs in three-dimensions in his or her mind and how to convey this information to other health care providers.
- •
The old cliché “the devil is in the details” is very appropriate to CMR imaging of complex CHD. This is especially true when imaging neonates, infants, and children. Spatial as well as temporal resolution details, for example, can make a huge difference between accurate diagnosis and misdiagnosis. Signal to noise and contrast to noise concerns are critical to successful imaging. The CMR imager must be familiar with the technical details of imaging and convey to others involved in the patient’s care both the differential diagnosis of the images as well as the potential for artifact.
- •
Knowing the limitations of CMR and the advantages and disadvantages of other modalities is vital in the evaluation of complex CHD. In general a combination of CMR and other techniques are necessary for the complete care of the patient.
In 1971 Mitchell et al. defined congenital heart disease (CHD) as a “gross structural abnormality of the heart or intrathoracic great vessels that is actually or potentially of functional significance.” Although this definition is problematic in simple CHD (e.g., patients with persistent fifth aortic arch or persistent left superior vena cava, both of which are not functionally significant), it fits complex CHD very well. Therefore this definition can include D-looped transposition of the great arteries (TGA) (i.e., segments [S,D,D]) as well as corrected TGA (i.e., segments [S,L,L]) without other lesions such as ventricular septal defect; patients with corrected TGA may not have any “actual” functional significance, but the potential for systemic right ventricular failure is always present. For this chapter on complex CHD, the Mitchell et al. definition will be used.
CHD itself is fairly common and complex CHD, although not as common as simple CHD, makes up a significant portion of young patients who present to the physician for care. In 2002, Hoffman and Kaplan reported the results of a survey of the literature on the incidence of CHD and found the average incidence of all these studies to be 9596 of one million live births (excluding nonstenotic bicuspid aortic valve, silent patent ductus arteriosus, and isolated partial anomalous pulmonary venous connections). Although complex CHD does not equate to cyanotic CHD, this literature review estimated the incidence of cyanotic CHD at 1391 per million live births (14.5% of all CHD). The New England Regional Infant Care Program (NERICP), however, estimated the incidence of “severe” CHD at 2033 per million live births with 888 being cyanotic. Clinicians, however, report that their patient population with these types of lesions requires a disproportionate share of their time (at least 50%). In addition, because of advances in medical care, more and more patients with complex CHD are living to adulthood and will need the care of adult and pediatric cardiologists; from 1985 to 2000, the prevalence of “severe” CHD increased significantly to such a degree that in 2000, there were equal number of children and adults alive with this type of heart disease.
Cardiac magnetic resonance (CMR) can play an important role in the medical and surgical management of complex CHD. Besides being a noninvasive technique without ionizing radiation, with regard to complex CHD, it has a wide field of view, which is important in determining the myriad of abnormal connections and anomalous structures that may be present both intracardiac and extracardiac. Because imaging extracardiac structures with CMR is routine (e.g., trachea, liver, etc.), the intricate relationship between cardiovascular structures and extracardiac structures can be evaluated to aid in surgical reconstruction. Along these lines, three-dimensional CMR imaging also is used in both the preoperative and postoperative surgical evaluation of these patients.
Another major strength of CMR as it relates to complex CHD is the ability to assess ventricular function and physiology. Ventricular volumes, mass, and ejection phase parameters of function, for example, can be very precisely calculated by CMR using cine techniques independent of geometric assumptions; this is clinically important in cases of complex CHD with the bizarre ventricular shapes as occurs in such as lesions as single ventricles, heterotaxy, or L-looped ventricles. Since these patients generally undergo surgery with cardiopulmonary bypass and deep hypothermic circulatory arrest (in some cases, multiple times), ventricular function is important to follow serially and accurately. In most instances, these images are the result of many heartbeats averaged together, which better reflect the true nature of ventricular function (as opposed to measurements that average three to four heartbeats such as in echocardiography). This “average” is embedded in the image, whereas the echocardiographer would need to examine many heartbeats and “average” these in his or her mind and come to a conclusion. CMR has been the gold standard in this area for nearly 20 years.
Because complex CHD can have complex flow patterns, CMR phase encoded velocity mapping can measure flows in any vessel to yield clinically important information such as shunt flow (e.g., Qp/Qs) or pulmonary flow in, for example, a patient with tricuspid atresia (S,D,S) with ventricular septal defect and pulmonic stenosis. This technique is also an average of many heartbeats, which is advantageous for the reasons stated above. Regurgitant fractions in lesions with valve insufficiency (e.g., pulmonary insufficiency after transannular patch for tetralogy of Fallot) can also be calculated in this manner.
Other CMR-based techniques are useful in the assessment of ventricular function and physiology of complex CHD. Time resolved, maximum intensity projection dimensional gadolinium sequence yields a set of three-dimensional images with temporal information and is useful to track the flow of blood from the venous side of the circulation onward. It can provide physiologic information on shunts; for example, in a case of a superior vena cava overriding the atrial septum with a sinus venosus atrial septal defect, both right and left atria would become signal intense. If there is a question of severe stenosis or discontinuity between branch pulmonary arteries, a small amount of gadolinium may be seen entering both right and left branches, confirming patency. Myocardial tagging, which noninvasively “labels” tissue, is useful in identifying regional ventricular wall dysfunction, which may be present in patients with coronary manipulations such as after arterial switch operation for TGA or after a Ross procedure for aortic stenosis. Myocardial perfusion imaging, performed by injecting gadolinium and observing the signal intensity in the myocardium, can be used for the assessment of these coronary lesions as well. Viability imaging, used by injecting gadolinium and waiting to image the myocardium (delayed enhancement technique), can identify regions of myocardial scar tissue (e.g., after surgery or after coronary manipulation) and foreign bodies (e.g., patch repair of an endocardial cushion defect). Finally, coronary imaging using the navigator technique can be used to determine coronary anomalies that can occur with complex CHD as well as to assess the coronaries after their manipulation.
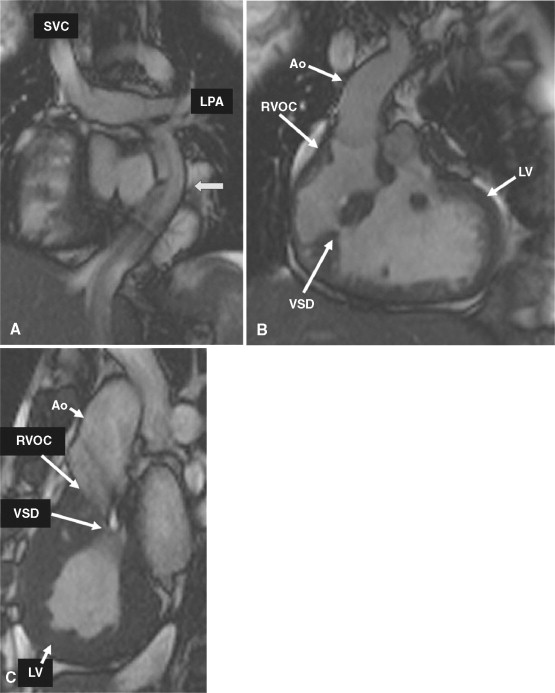
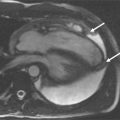
A 19-year-old patient presents for routine follow-up of her single ventricle lesion ( Figure 15-1 ). The anatomy is TGA (S,L,L) with dextrocardia, tricuspid atresia and a right ventricular outflow chamber who is after Fontan. Systemic blood flow enters the pulmonary arteries passively from the superior vena cava and inferior vena cava via the Fontan baffle. Pulmonary venous blood enters the left atrium followed by the right-sided left ventricle, across ventricular septal defects into the left-sided right ventricular outflow chamber and then the aorta. CMR found obstruction to flow across the ventricular septal defects with a peak instantaneous gradient of 85 mm Hg; at cardiac catheterization, the peak to peak gradient was found to be 50 mm Hg (left ventricular systolic pressure of 170 mm Hg and the right ventricular outflow chamber of 120 mm Hg).