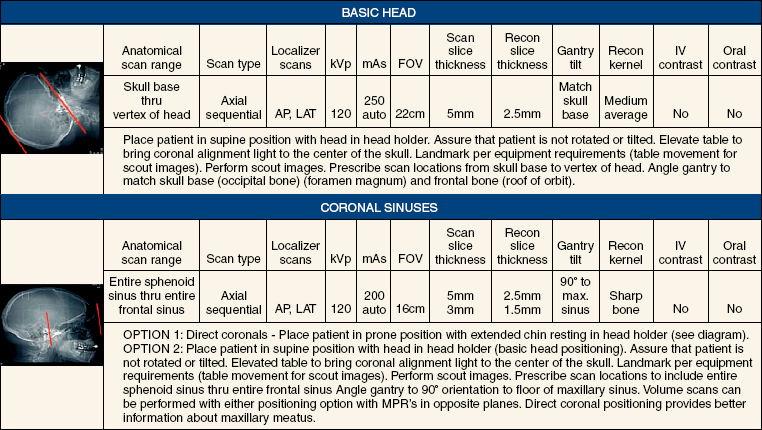
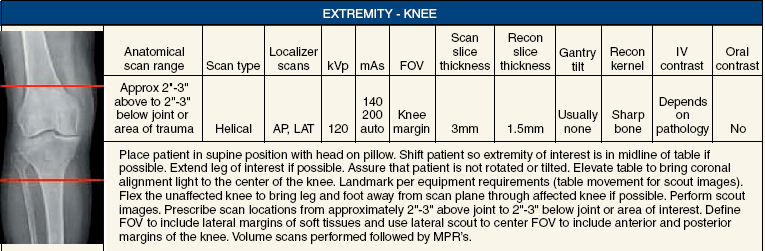
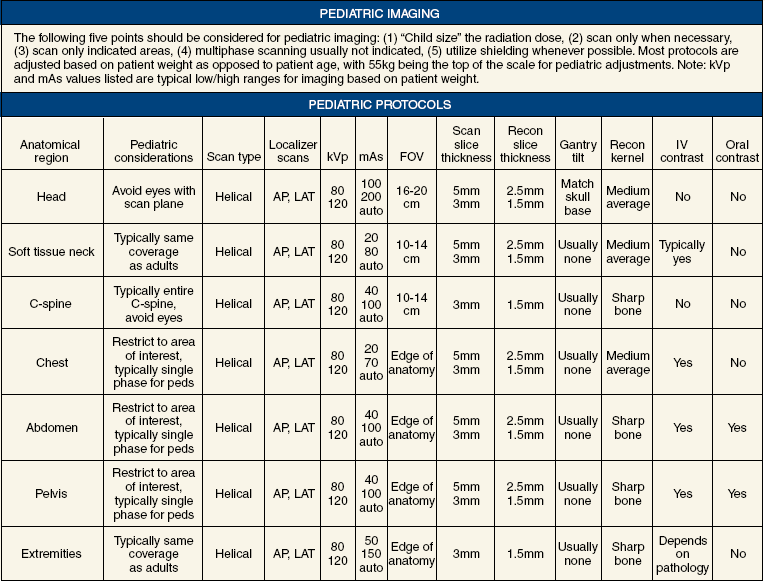
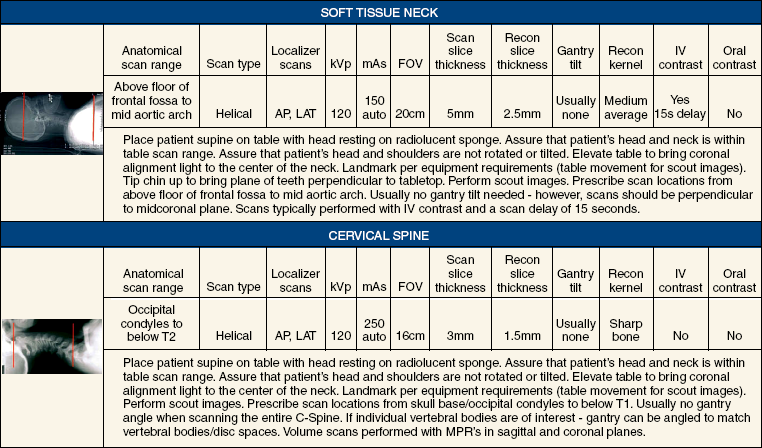
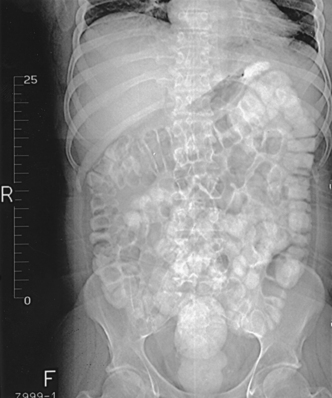
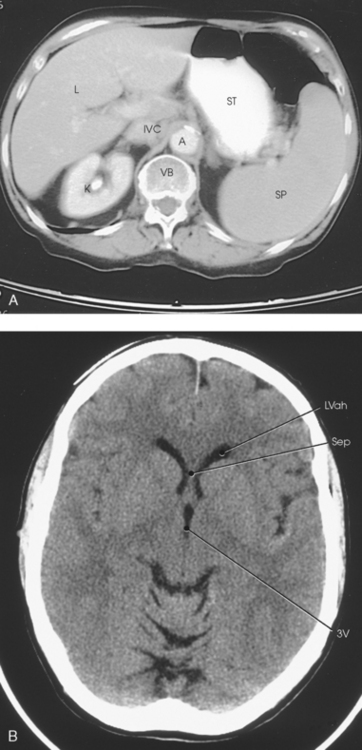
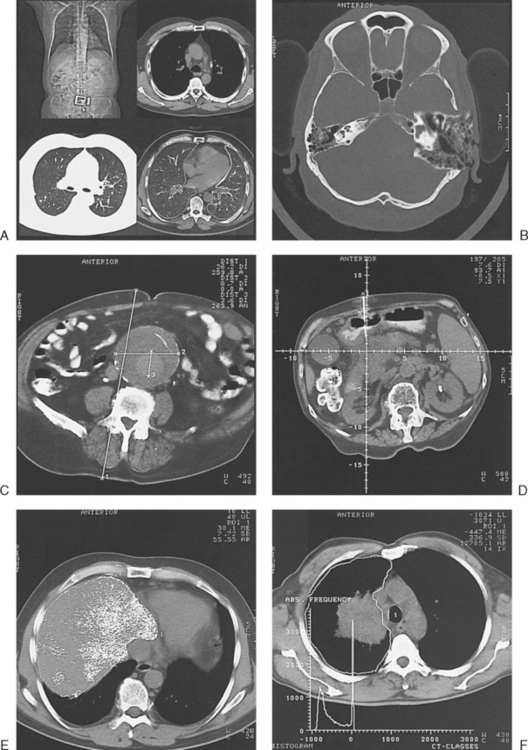
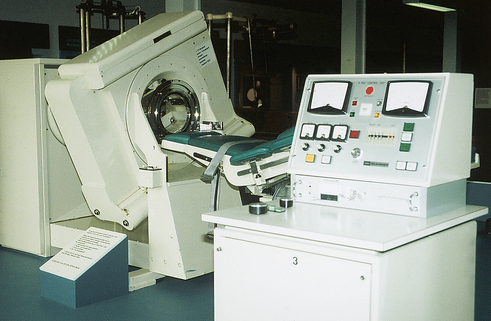
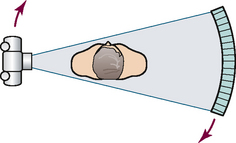
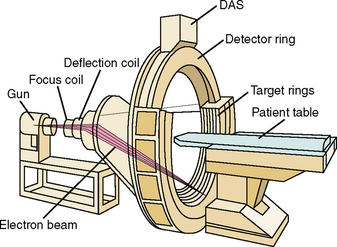
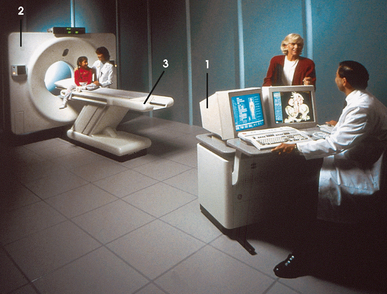
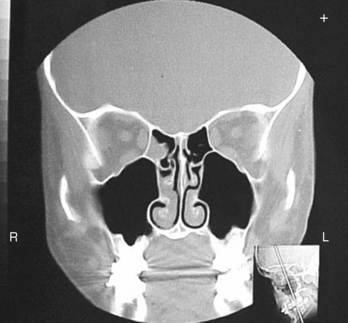
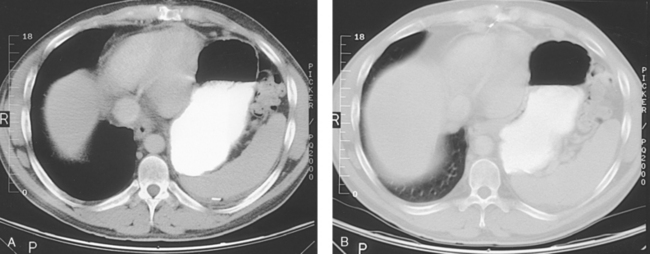
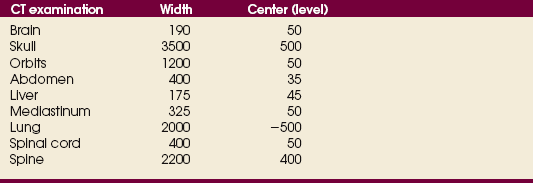
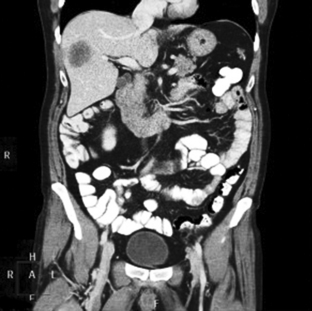
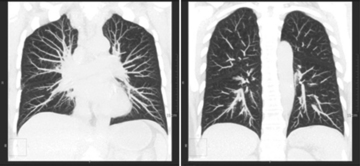
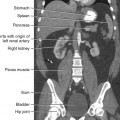
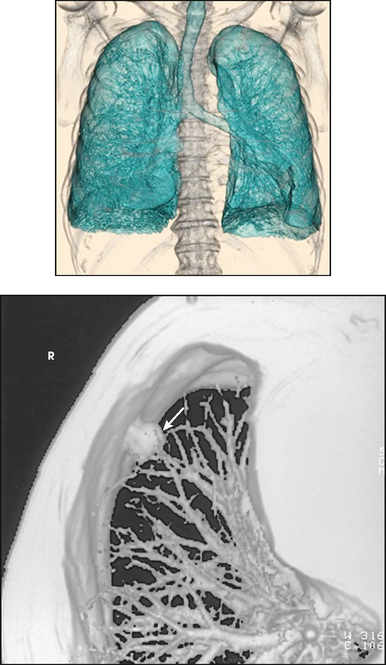
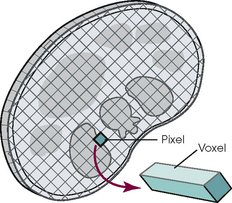
31 Basic computed tomography examination protocols Fundamentals of computed tomography Computed tomography and conventional radiography Computed tomography scanner generation classifications Factors affecting image quality Computed tomography and radiation dose Comparison of computed tomography and magnetic resonance imaging Computed tomography (CT)* is the process of creating a cross-sectional tomographic plane of any part of the body (Fig. 31-1). For CT, a patient is scanned by an x-ray tube rotating around the body part being examined. A detector assembly measures the radiation exiting the patient and feeds back the information, referred to as primary data, to the host computer. After the computer has compiled and calculated the data according to a preselected algorithm, it assembles the data in a matrix to form an axial image. Each image, or slice, is displayed in a cross-sectional format. When a conventional x-ray exposure is made, the radiation passes through the patient and produces an image of the body part. Frequently, body structures are superimposed (Fig. 31-2). Visualizing specific structures requires the use of contrast media, varied positions, and usually more than one exposure. Localization of masses or foreign bodies requires at least two exposures and a ruler calibrated for magnification. In the digital radiograph of the abdomen shown in Fig. 31-3, high-density bone and low-density gas are seen, but many soft tissue structures, such as the kidneys and intestines, are not clearly identified. Contrast media are needed to visualize these structures. A CT examination of the abdomen would show all of the structures that lie within the slice. In Fig. 31-4, A, the liver, stomach, kidneys, spleen, and aorta can be identified. In addition to eliminating superimposition, CT is capable of differentiating among tissues with similar densities. This differentiation of densities is referred to as contrast resolution. The improved contrast resolution with CT compared with conventional radiography is due to a reduction in the amount of scattered radiation. Fig. 31-4, B, is an axial image of the brain that differentiates the gray matter from the white matter and shows bony structures and cerebrospinal fluid within the ventricles. Because CT can show subtle differences in various tissues, radiologists are able to diagnose pathologic conditions more accurately than if they were to rely on radiographs alone. Because the image is digitized by the computer, numerous image manipulation techniques can be used to enhance and optimize the diagnostic information available to the physician (Fig. 31-5). CT was first performed successfully in 1970 in England at the Central Research Laboratory of EMI, Ltd. Hounsfield, an engineer for EMI, and Cormack, a nuclear physicist from Johannesburg, South Africa, are generally given credit for the development of CT. For their research, they were awarded the Nobel Prize in Medicine and Physiology in 1979. After CT was shown to be a useful clinical imaging modality, the first full-scale commercial unit, referred to as a brain tissue scanner, was installed in Atkinson Morley’s Hospital in 1971. An early dedicated head CT scanner is shown in Fig. 31-6. Physicians recognized its value for providing diagnostic neurologic information, and its use was accepted rapidly. The first CT scanners in the United States were installed in June 1973 at the Mayo Clinic, Rochester, Minnesota, and later that year at Massachusetts General Hospital, Boston. These early units were also dedicated head CT scanners. In 1974, Ledley at Georgetown University Medical Center, Washington, D.C., developed the first whole-body scanner, which greatly expanded the diagnostic capabilities of CT. The early units, referred to as first-generation scanners, worked by a process known as translate/rotate. The tube produced a finely collimated beam, or pencil beam. Depending on the manufacturer, one to three detectors were placed opposite the tube for radiation detection. The linear tube movement (translation) was followed by a rotation of 1 degree. Scan time was usually 3 to 5 minutes per scan, which required the patient to hold still for extended periods. Because of the slow scanning and reconstruction time, the use of CT was limited almost exclusively to neurologic examinations. A CT image from a first-generation scanner is shown in Fig. 31-7. The third–generation scanners introduced a rotate/rotate movement, in which the x-ray tube and detector array rotate simultaneously around the patient. An increase in the number of detectors (>750) and their arrangement in a “curved” detector array considerably improved image quality (Fig. 31-8). Scan times were decreased to 0.35 to 10 seconds per slice, which made the CT examination much easier for patients and helped decrease motion artifact. Advancements in computer technology also decreased image reconstruction time, substantially reducing examination time. Most current scanners are third-generation configurations with one of the following technical variations: • Helical CT, single-slice helical CT (SSHCT). Slip-ring technology allows 360-degree continuous rotation of tube and detector. Reduces scan times to subsecond per slice. • Multislice detectors (MSHCT or MDCT). Increase in number of detector rows allows multiple slices to be taken in one rotation. As detector rows increase, the fan beam geometry of the x-ray beam has been adapted. Began with two-slice scanners and quickly moved to four-slice and more. • Volume CT (VCT). Multislice scanners with 64 detector rows or more. The x-ray beam geometry must be a cone-beam configuration to accommodate the increased length of the scanner. • Flat-panel CT (FP-CT or FD-CT). A detector plate similar to plates used in digital radiography (DR) replaces the typical detector configuration. In dedicated breast units, the tube and detector travel a full 360 degrees. In other applications, the unit functions more like a C-arm fluoroscopy unit in which the tube and detector do not travel in a full 360 degrees. Scanners provide excellent spatial resolution but lower contrast resolution. The fourth-generation scanners introduced the rotate-only movement in which the tube rotates around the patient, but the detectors were in fixed positions, forming a complete circle within the gantry (Fig. 31-9). The use of stationary detectors required greater numbers of detectors to be installed in a scanner. Fourth-generation scanners tended to yield a higher patient dose per scan than previous generations of CT scanners. Fig. 31-9 Rotate-only movement: tube movement with stationary detectors of a fourth-generation scanner. The fifth-generation scanners are classified as high-speed CT scanners because of millisecond acquisition times. These scanners are electron-beam scanners (EBCT) in which x-rays are produced from an electron beam in a fan beam configuration that strikes stationary tungsten target rings (Fig. 31-10). The detector rings are in a ±210-degree arc. These scanners were primarily used for cardiac studies. The sixth-generation scanners are dual-energy source (two x-ray tubes) (DSCT, DE-CT) that have two sets of detectors that are offset by 90 degrees. These DSCT scanners provide improved temporal resolution needed for imaging moving structures such as the heart (Fig. 31-11). The original DSCT scanners had several technical challenges and were not widely used. The latest DSCT scanners have solved the technical issues, however, and offer dual-energy capabilities between the two CT tubes. This technology allows a marked decrease in patient radiation dose. Fig. 31-11 Dual-source CT scanner (DSCT) configuration. This is considered a sixth-generation scanner. Most scanners in use today are a third-generation variation that have 4 to 320 rows of detectors in a single array. This increase in numbers of detector rows has increased the length of the detector, which requires the x-ray beam to be cone-shaped to encompass the full detector array. This is a change from the original third-generation fan beam. The flat panel detector also requires cone-beam geometry. The increased detector size and the cone-beam geometry pose various challenges in maintaining image quality, but this is a discussion that is too involved for this chapter. The digital image is an array of numbers arranged in a grid of rows and columns called a matrix. A single square, or picture element, within the matrix is called a pixel. The slice thickness gives the pixel an added dimension called the volume element, or voxel. Each pixel in the image corresponds to the volume of tissue in the body section being imaged. The voxel volume is a product of the pixel area and slice thickness (Fig. 31-12). The field of view (FOV) determines the amount of data to be displayed on the monitor. Fig. 31-12 CT image is composed of a matrix of pixels, with each pixel representing a volume of tissue (voxel). Each pixel within the matrix is assigned a number that is related to the linear attenuation coefficient of the tissue within each voxel. These numbers are called CT numbers or Hounsfield units. CT numbers are defined as a relative comparison of x-ray attenuation of a voxel of tissue with an equal volume of water. Water is used as reference material because it is abundant in the body and has a uniform density; water is assigned an arbitrary value of 0. Tissues that are denser than water are given positive CT numbers, and tissues with less density than water are assigned negative CT numbers. The scale of CT numbers ranges from −1000 (air/gas) to +3000 (dense bone). Average CT numbers for various tissues are listed in Table 31-1. TABLE 31-1 Average Hounsfield units (HU) for selected substances The three major components of the CT scanner are shown in Fig. 31-13. Because each component has several subsystems, only a brief description of their main functions is provided in the following sections. The gantry can be tilted forward or backward up to 30 degrees to compensate for body part angulation. The opening within the center of the gantry is termed the aperture. Most apertures are about 28 inches (71.1 cm) wide to accommodate a variety of patient sizes as the patient table advances through it. For certain head studies, such as studies of facial bones, sinuses, or the sella turcica, a combination of patient positioning and gantry angulation results in a direct coronal image of the body part being scanned. Fig. 31-14 shows a typical direct coronal image of the paranasal sinuses. The operator’s console (Fig. 31-15) is the point from which the technologist controls the scanner. A typical console is equipped with a keyboard for entering patient data and a graphic monitor for viewing the images. Other input devices, such as a touch display screen and a computer mouse, may also be used. The operator’s console allows the technologist to control and monitor numerous scan parameters. Radiographic technique factors, slice thickness, table index, and reconstruction algorithm are some of the scan parameters that are selected at the operator’s console. Before starting an examination, the technologist must enter the patient information. A keyboard is still necessary for some functions. Usually the first scan program selected is the scout program from which the radiographer plans the sequence of axial scans. An example of a typical scout image is seen in Fig. 31-3. The operator’s console is also the location of the monitor, where image manipulation takes place. Most scanners display the image on the monitor in a 1024 matrix interpolated by the computer from the 512 reconstructed images. Because of the digital nature of the CT image data, image manipulation can be performed to enhance the appearance of the image. One of the most common image processing techniques is called windowing, or gray-level mapping. This technique allows the technologist to alter the contrast of the displayed image by adjusting the window width and window level. The window width is the range of CT numbers that are used to map signals into shades of gray. Basically, the window width determines the number of gray levels to be displayed in the image. A narrow window width means that there are fewer shades of gray, resulting in higher contrast. Likewise, a wide window width results in more shades of gray in the image, or a longer gray scale. The window level determines the midpoint of the range of gray levels to be displayed on the monitor. It is used to set the center CT number within the range of gray levels being used to display the image. The window level should be set to the CT number of the tissue of interest, and the window width should be set with a range of values that would optimize the contrast between the tissues in the image. Fig. 31-16 shows an axial image seen in two different windows: a standard abdomen window and a bone window adjusted for the spine. The gray level of any image can be adjusted on the monitor to compensate for differences in patient size and tissue densities or to display the image as desired for the examination protocol. Examples of typical window width and level settings are listed in Table 31-2. These settings are averages and usually vary by machine. The level, although an average, is approximately the same as the CT numbers expected for the tissue densities. Another advantage of the digital nature of the CT image is the ability to reconstruct the axial images into coronal, sagittal, or oblique body planes without additional radiation to the patient. Image reconstruction in various planes is accomplished by stacking multiple contiguous axial images, creating a volume of data. Because the CT numbers of the image data within the volume are already known, a sectional image can be generated in any desired plane by selecting a particular plane of data. This postprocessing technique is termed multiplanar reconstruction (MPR). A coronal reconstruction from axial images is seen in Figs. 31-17 and 31-18. Fig. 31-17 shows a coronal image of the abdomen (note the liver lesion), and Fig. 31-18 shows coronal images of the lungs displayed with a lung window width and window level. MPRs may also be performed in what is referred to as curved planar reformations to visualize structures better. Fig. 31-19 shows an axial image and oblique reformation of the mandible from the axial images The original CT studies were used primarily for diagnosing neurologic disorders. As scanner technology advanced, the range of applications was extended to other areas of the body. The most commonly requested procedures involve the head, chest, and abdomen. CT is the examination of choice for head trauma; it clearly shows skull fractures and associated subdural hematomas. CT examinations of the head are one of the first tests performed on patients being evaluated for stroke or cerebrovascular accident where evidence of hemorrhage must be ruled out. CT imaging of the central nervous system can show infarctions, hemorrhage, disk herniations, craniofacial and spinal fractures, and tumors and other cancers. CT imaging of the body excels at showing soft tissue structures within the chest, abdomen, and pelvis. Among the abnormalities shown in this region are metastatic lesions, aneurysms (Fig. 31-20), abscesses, and fluid collections from blunt trauma.
COMPUTED TOMOGRAPHY
Fundamentals of Computed Tomography
Computed Tomography and Conventional Radiography
Historical Development
Computed Tomography Scanner Generation Classifications
Technical Aspects
Substance
HU
Air
−1000
Lungs
−250 to −850
Fat
−100
Orbit
−25
Water
0
Cyst
−5 to +10
Fluid
0 to +25
Tumor
+25 to +100
Blood (fluid)
+20 to +50
Blood (clotted)
+50 to +75
Blood (old)
+10 to +15
Brain
+20 to +40
Muscle
+35 to +50
Gallbladder
+5 to +30
Liver
+40 to +70
Aorta
+35 to +50
Bone
+150 to +1000
Metal
+2000 to +4000
System Components
GANTRY AND TABLE
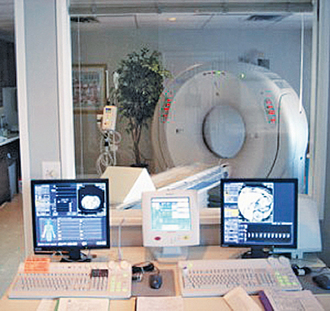
OPERATOR’S CONSOLE
OTHER COMPONENTS
Workstation for image manipulation and multiplanar reconstruction
Diagnostic Applications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine