Disorders of the corpus callosum
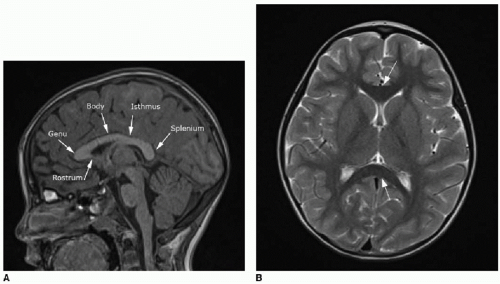
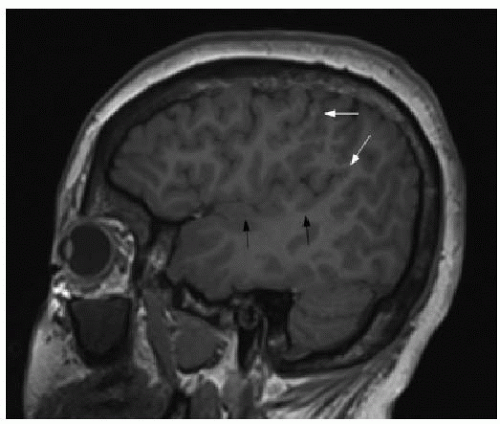
The corpus callosum is a large midline bundle of crossing white matter fibers connecting the cerebral hemispheres. It develops from approximately 8 weeks through 18 weeks of fetal gestation. The corpus callosum grows primarily from anterior to posterior, with the genu forming first, followed by the anterior body, posterior body, and splenium. The rostrum, however, forms after the splenium, between 18 and 20 weeks of gestational age (
Fig. 30.1).
2 Partial or complete malformation (agenesis) of the corpus callosum has an incidence of 1 in 4,000 live births.
3 Although agenesis of the corpus callosum can be an isolated finding, callosal malformations are more commonly seen in conjunction with other brain anomalies, including Chiari malformation, encephalocele, migrational anomalies, holoprosencephaly (HPE), and malformations of the brainstem or cerebellum.
3,4 The prognosis depends on all associated abnormalities and underlying genetic influences. Although isolated callosal malformations may be asymptomatic, seizures, microcephaly, and developmental delays are frequently observed.
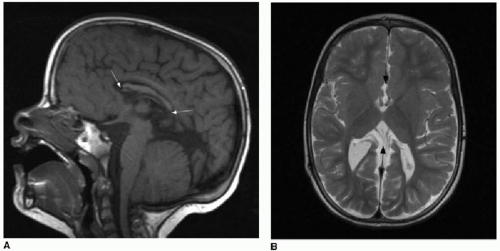
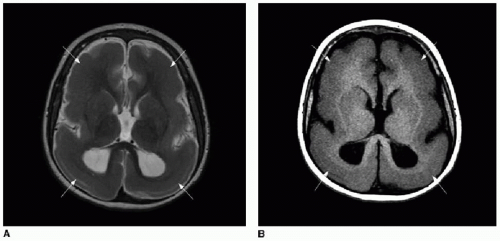
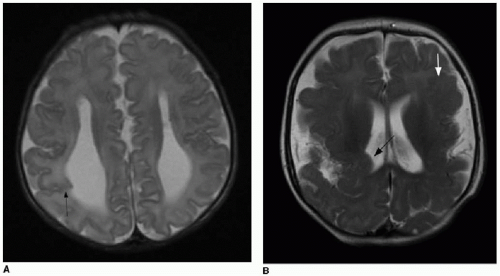
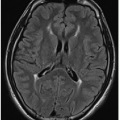
5Partial dysgenesis most commonly manifests with absence of the splenium and a portion of the mid- to posterior body (
Fig. 30.2A, B). Diffuse or segmental callosal thinning may result secondary to a vascular or inflammatory insult that destroys white matter volume (
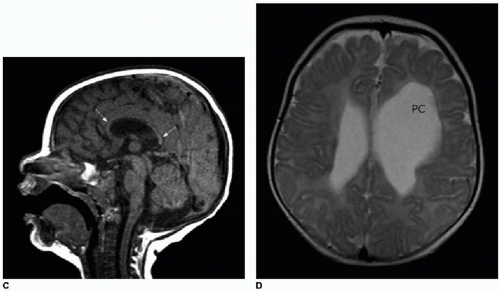
Fig. 30.2C, D). A rare association with callosal agenesis or dysgenesis is a midline lipoma, which is presumed to arise from abnormal differentiation of the meningeal precursor cells.
6 Of note, midline lipomas can be present even in
the setting of a normally formed corpus callosum, and they have no clinical implication (
Fig. 30.3).
Migrational abnormalities
Normal cerebral cortical development depends on neuronal cell proliferation, differentiation, migration, and organization.
7, 8 and 9 Disruption of any of these processes may occur in the context of genetic anomalies, congenital infections such as cytomegalovirus or toxoplasmosis, ischemic injury, and toxin exposure or may occur sporadically. Cellular proliferation and migration may be either insufficient or exuberant, resulting in very different appearances of the brain.
9 Table 30.1 summarizes the various malformations included within the category of migrational anomalies.
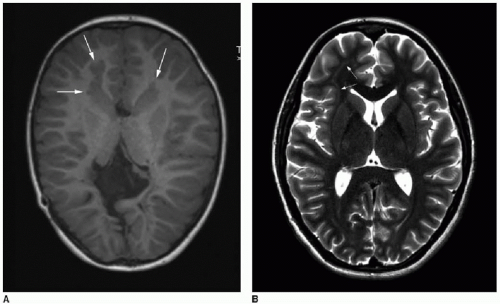
Lissencephaly, or “smooth brain,” is a term that applies to a number
of diffuse proliferative or migrational disorders characterized by abnormal simplification of the cortical convolutions (
Fig. 30.4).
10,11 Agyria refers to absence of cortical gyri, whereas pachygyria refers to abnormally broad cerebral gyri; these terms also fall within the category of lissencephaly. A number of genetic defects causing lissencephaly have been identified.
11 While diffuse migrational anomalies present with severe developmental delay, intractable seizures, and hypertonia, the focal malformations tend to present with medical refractory seizures, and we will focus on these entities here.
Gray matter heterotopias are nodules or masses of abnormal tissue that follow gray matter signal on MR sequences and are histologically characterized by a combination of normal neurons and glial cells.
7,9 These may be found in the periventricular or subcortical white matter (
Fig. 30.5). Periventricular heterotopias tend to manifest with focal seizures, whereas subcortical heterotopias more commonly are associated with partial complex and tonic-clonic seizures.
7 The more extensive the heterotopia, the more likely the child will be affected by developmental delays in
addition to seizures.
7 A diffuse form of gray matter heterotopia is called subcortical band heterotopia (SBH), wherein a thick band of tissue isointense to gray matter replaces a variable volume of white matter, and only a thin rim of white matter is evident between the heterotopic tissue and the cortex (
Fig. 30.4). SBH falls into the category of lissencephaly, and its clinical severity depends on the extent of cortical abnormalities, band thickness, and ventricular enlargement.
11
Focal cortical dysplasia (FCD) has recently been classified according to the histologic cortical laminar structure and architectural disruption, cell composition, and presence of associated destructive lesions.
12 Types I and II are isolated lesions that are both characterized by abnormal cortical lamination. They are differentiated based on absence (type I) or presence (type II) of dysmorphic neurons. Type II dysplasias may also have eosinophilic balloon cells, which were first described in 1971 and may be grossly abnormal glial cells.
13 Type III lesions are focal areas of cortical lamination abnormalities associated with a destructive lesion such as hippocampal sclerosis, glial tumor, vascular malformation, or a focal insult acquired in fetal or early life such as infection or infarction.
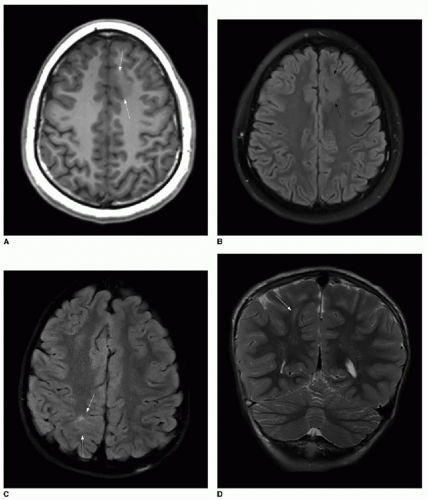
12 Identification of FCD on brain MRI requires careful scrutiny of high-resolution images. The hallmarks of type I FCD include cortical thickening and loss of a distinct border between gray and white matter (
Fig. 30.6A, B). Type II is more readily identified on MR. The
transmantle sign describes a radially oriented linear or conical subcortical T2 hyperintensity, reflecting the radial extension of balloon cells and ectopic neurons from the cortex into the affected white matter (
Fig. 30.6C, D). This sign can be useful in differentiating types I and II FCD.
14 Surgical resection of FCD results in a seizure-free outcome in 33% to 75% of cases, depending on center-specific selection criteria for surgical eligibility.
14,15
Polymicrogyria (PMG) accounts for approximately 20% of all cortical malformations observed. It is characterized by an excessive number of small cortical gyri, with distortion of the expected gyral and sulcal pattern.
8,16 The extent of PMG varies greatly, as does the range of clinical manifestations. When PMG is bilateral, it is most often found along the Sylvian fissures (
Fig. 30.7).
17 Several chromosome abnormalities are associated with PMG; the most common are the 1p36.3 microdeletion and the 22q11.2 microdeletion, both of which are associated with unilateral or bilateral perisylvian PMG.
17 Schizencephaly is a specific pattern of PMG defined as a full-thickness cleft in the brain.
18,19 These clefts must be lined by PMG to be called schizencephaly (
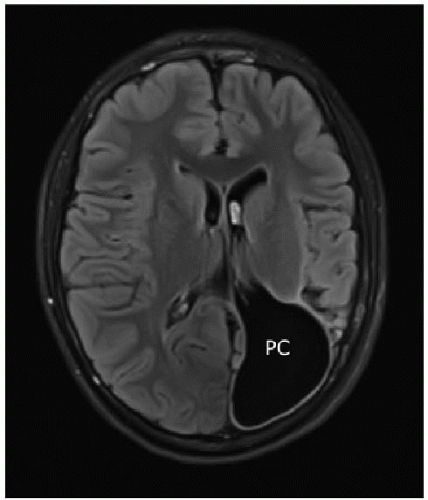
Fig. 30.8), as opposed to a porencephalic cyst (
Figs. 30.2D and
30.9), which can be thought of as an intraparenchymal cerebrospinal fluid (CSF) space lined by white matter or gliosis, resulting presumably from a fetal insult causing focal brain destruction.
17 Schizencephaly has been described as closed lip or open lip—if the cleft contains CSF density or signal, it is open lip, whereas closed-lip anomalies lack interposed CSF on imaging.
18,19 However, differentiation of the two types is generally not clinically relevant. Neuronal migrational abnormalities can be associated with other brain malformations, so it is important to search carefully for other findings.