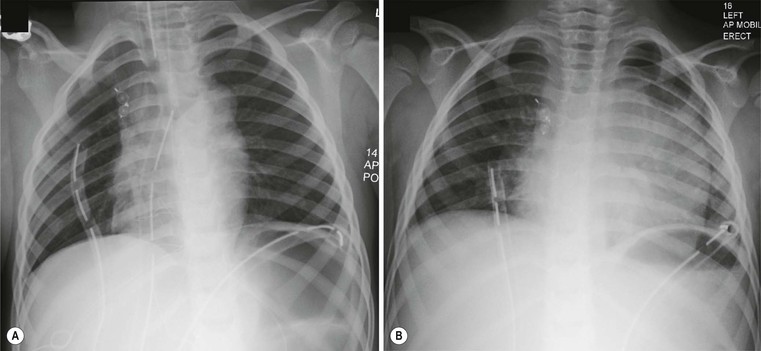
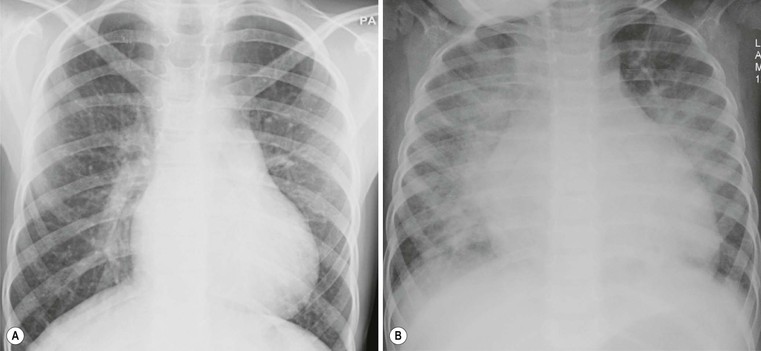
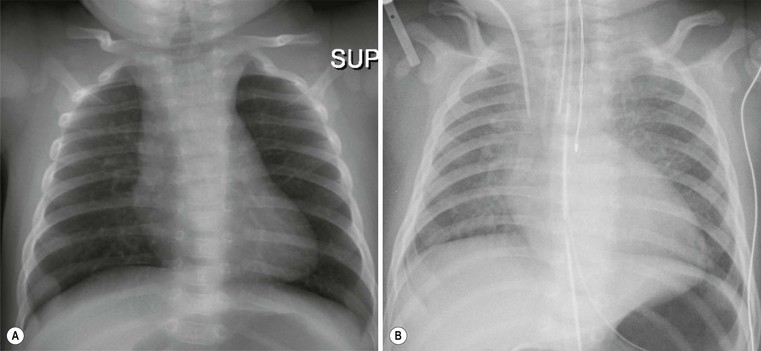
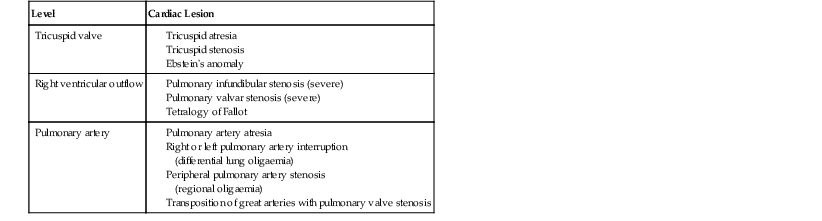
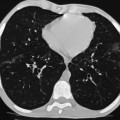
Andrew M. Taylor, Michael A. Quail Congenital heart disease (CHD), although rare, with an incidence of 8 per 1000 births, has increased in prevalence due to the success of surgical and medical management in childhood. A significant proportion of patients with repaired CHD surviving to adulthood fall under the care of cardiologists outside tertiary centres for congenital cardiac care. Specialist cardiovascular and general radiologists require an understanding of the underlying morphological abnormalities and their physiology, methods of repair and how potential complications may be detected and assessed in their practice, using appropriate imaging techniques, such as ultrasound (US), cardiac magnetic resonance (CMR) and computed tomography (CT). Congenital heart disease is any developmental malformation of the heart. The spectrum of disease falling into this classification ranges from simple lesions, for example bicuspid aortic valve, through to more complex diseases involving single ventricle lesions, such as hypoplastic left heart syndrome (HLHS). The developmental biology and genetic basis of CHD is under investigation, but apart from a few well-known associations—for example Di George syndrome (interrupted aortic arch, tetralogy of Fallot, truncus arteriosus), Down’s syndrome (atrioventricular, ventricular and atrial septal defects), and Turner’s syndrome (bicuspid aortic valve and coarctation)—the cause of the majority of clinical disease is unknown. The clinical presentation of CHD in infancy may be dominated by a number of physiological states. 3. Pulmonary venous congestion: Obstruction to pulmonary venous return results in increased pulmonary venous pressure (elevated pulmonary capillary wedge pressure); at progressively higher transvascular gradients, colloid osmotic pressure is exceeded and extravasation of fluid into the interstitial and alveolar space occurs. Obstruction may occur in the pulmonary venous pathway (total anomalous pulmonary venous connection, TAPVD), in the atrium (cor triatriatum) or at the level of the left ventricular inflow (supravalvular, valvular or subvalvular mitral stenosis or mitral regurgitation). Pulmonary venous congestion may also occur as a function of elevated left atrial pressure secondary to LV diastolic dysfunction: increased LV end diastolic pressure (valve disease, aortic coarctation, myocardial disease). The degree of pulmonary venous hypertension determines the clinical presentation. Patients with severe obstruction may present with hypoxia, cyanosis and dyspnoea due to pulmonary oedema, whilst patients with less severe obstruction may remain pink but may present later with failure to thrive. Most adult patients with CHD are survivors from childhood. This group may present with an interesting array of problems related to residual lesions or deteriorations of their initial repair or palliation (heart failure, valve regurgitation, conduit stenosis, baffle leaks). They require lifelong surveillance for anticipated problems arising from the ‘unnatural history’ of their underlying disorder. New presentations of CHD continue beyond infancy into adulthood, usually because the underlying disorder has not yet produced symptoms. Common lesions include: left-to-right shunts, such as ASDs, VSDs or partial anomalous pulmonary venous drainage which only begin to be symptomatic in older patients; milder forms of LV or aortic obstruction such as coarctation of the aorta or valvular aortic stenosis which did not compromise systemic perfusion may progress and become symptomatic in later childhood or adulthood; and milder forms of RV obstruction such as pulmonary stenosis. Clearly, life-limiting conditions such as parallel circulation, significant shunts, severe intracardiac mixing or compromised systemic perfusion, would not normally be expected beyond childhood. Numerous morphological abnormalities may be responsible for the physiological phenomena described above, and although initial management of an infant simply requires correct classification of the initial physiological pattern, subsequent surgical correction and medical management requires a precise anatomical diagnosis. The potential intrinsic complexity of CHD necessitates a systematic scheme of nomenclature that captures precisely the unique anatomy of each patient: this is called sequential segmental analysis. Using this approach, the clinician describes how the components of the heart and blood vessels are connected. This entails describing atrial situs (location of the atrial chambers and whether they are of left or right morphology), atrioventricular connections, ventriculo-arterial connections and other associated lesions in turn. Any cross-sectional imaging investigation may be used for this purpose, but transthoracic ultrasound is most commonly used for routine inpatient and outpatient assessment. In more complex lesions or when ultrasound provides an inadequate assessment (e.g. poor acoustic windows), CMR represents a powerful non-invasive technique giving morphological and haemodynamic information that ultrasound alone cannot provide. Atrial situs is determined by an assessment of the morphology of each atrial appendage. Correct identification of the atrium allows the subsequent determination of the atrioventricular connection. The atrial appendages are the most consistent feature of the atrial mass; indeed, the venous attachments to each atrial chamber can form a variety of combinations. The right atrial appendage is a triangular shape, with a broad base and prominent pectinate muscles which extend around the right atrioventricular valve, whilst the left atrial appendage is a more elongated, tubular structure, and it has less extensive pectinate muscles which are confined within the appendage. The most common lesions involve inversion of situs, or isomerism of the left or right atrial appendages. The non-cardiac thoracic and abdominal organs usually (but not always) demonstrate a similar ‘sidedness’ to that of the atrial chambers. In the normal heart the morphological right atrium is located to the right of the morphological left atrium (situs solitus). The right lung is trilobed, with a shorter, early-branching bronchus, and the left lung is bilobed. In addition, the inferior caval vein (IVC) is to the right of the abdominal aorta, with a right-sided liver and left-sided spleen. In situs inversus the mirror image of the normal anatomy is present. Isomerism of the left atrial appendages is usually associated with bilateral bilobed lungs, polysplenia and IVC interruption. Isomerism of the right atrial appendages is usually associated with bilateral trilobed lungs, asplenia and a midline liver. In isomeric lesions there is often a common atrioventricular junction (instead of two separate and offset left and right junctions) with varying degrees of atrioventricular septal defect (AVSD). Gut malrotation is associated with both right- and left-sided isomerism. All of these abnormalities can be determined with CMR, particularly 3D balanced steady-state free precession (b-SSFP) technique. Determination of ventricular morphology allows analysis of atrioventricular (AV) and ventriculo-arterial connections. An AV connection is described as ‘concordant’ when the atria is connected to the expected ventricle (i.e. left atrium with left ventricle and right atrium with right ventricle); ‘discordant’ if the left atrium is connected to the right ventricle and right atrium to the left ventricle; ‘ambiguous’ if there is isomerism of the atrial appendages (e.g. two morphologically right atria connected to a left and right ventricle, respectively (one connection is concordant, the other discordant) ); and finally ‘univentricular’ if both atria predominately connect to a single ventricle. Irrespective of atrioventricular concordance the AV valve is always concordant with the ventricle, i.e. the tricuspid valve connects to the morphological right ventricle and the mitral valve connects to the morphological left ventricle. The septal insertion of the tricuspid valve is more apical (apically ‘offset’) than that of the mitral valve, and allows determination of the ventricular morphology. The muscular structure of the ventricles also differs, the RV being more trabeculated than the LV, with a muscular infundibulum and mid-ventricular ‘moderator band’. Although they are different in normal subjects, the size, shape and degree of trabeculation of the ventricles are not good indicators of ventricular origin, as all are dependent on load effects. Description of ventriculo-arterial connections represents the final element of sequential segmental analysis. This entails describing how each great vessel is connected to its respective ventricle. A ventriculo-arterial connection may be concordant (RV–PA, LV-aorta), discordant (RV–aorta, LV–PA), double outlet (e.g. RV–PA and aorta) or single outlet (e.g. LV and RV to common arterial trunk). The aorta and pulmonary arteries are defined by their typical branching patterns. Three-dimensional b-SSFP and contrast-enhanced MR angiography (MRA) techniques are particularly useful in determining the arrangement of the great vessels and the connections with their respective ventricles. Other abnormalities to be considered include: abnormal venous connections, ASD, VSD, AVSD and valve abnormalities. In general, most congenital cardiac lesions are single abnormalities that are easily described. However, almost any combination of abnormalities and connections can occur, and using the sequential segmental analysis method, the description of all conceivable combinations and diagnoses is possible. For more advanced reading, the reader is referred to the textbook Paediatric Cardiology by Anderson and colleagues.1 In addition to determining the morphology of underlying lesions, an assessment of their physiological impact on heart function is necessary. It is helpful to briefly consider a few parameters relating to normal cardiac function, which are commonly calculated by techniques such as CMR. Imaging is fundamental to the diagnosis of CHD and is required at all stages of patient care. An ideal non-invasive investigation for imaging of CHD should be able to accurately and reproducibly delineate all aspects of the anatomy, including intracardiac abnormalities and abnormalities of extracardiac vessels, evaluate physiological consequences of CHD such as measurement of blood flow and pressure gradients across stenotic valves or blood vessels, be cost-effective and portable, provide data from fetal life to adulthood, not cause excessive discomfort and morbidity, and not expose patients to harmful effects of ionising radiation. No single investigation has fulfilled these entire requirements, and in the delivery of a CHD service, the imaging techniques discussed below play an important complementary role. Ultrasound is the initial imaging investigation used in the evaluation of patients with suspected CHD and should always be performed before other techniques are used. In most patients, ultrasound alone provides sufficient information to complete the diagnostic evaluation using a sequential segmental and functional analysis. In UK clinical practice, paediatric cardiologists have traditionally performed ultrasound. However, more recently neonatologists and radiologists have begun to use ultrasound in patients with suspected CHD where paediatric cardiology services are not immediately available. Cardiac anaesthetists also increasingly perform perioperative assessment using transoesophageal ultrasound. For a more comprehensive discussion of ultrasound in congenital heart disease, the reader is referred to Lai et al.2 As previously alluded, CMR probably provides the most comprehensive assessment available from a single non-invasive imaging investigation, but its immobility, cost, and limited availability constrain its general applicability. In our clinical practice it is used to define the morphology and physiology of the most complex CHD cases as well as providing routine surveillance for patients with repaired CHD such as tetralogy of Fallot and transposition of the great arteries. Extracardiac anatomy, including the great arteries and systemic veins, can be delineated with high spatial resolution. Vascular and valvular flow can be assessed, shunts can be quantified, and myocardial function can be measured accurately with high reproducibility, regardless of ventricular morphology. Finally, CMR provides high-resolution, isotropic, three-dimensional datasets. This allows for reconstruction of data in any imaging plane, facilitating visualisation of complex cardiac anomalies, without the use of ionising radiation. The majority of CMR images are acquired using cardiac (ECG) gating during a single breath-hold to reduce the artefacts associated with cardiac and respiratory motion. For a complex case, CMR is performed over approximately 1 hour, though this time can be considerably reduced if a focused question is being addressed or by the incorporation of newer real-time sequences. Imaging sequences can be broadly divided into: • Phase-contrast imaging, where velocity information is encoded for quantification of vascular flow. All these sequences can be acquired in a single breath-hold, reducing the overall time in the CMR machine, and enabling the acquisition of accurate data in the majority of patients. Importantly, ‘white-blood’ cine images can be acquired in a continuous short-axis stack along the heart, enabling accurate quantification of right and left ventricular function. Imaging should be performed in the presence of a cardiovascular MRI clinician in conjunction with an MRI technician to ensure that the appropriate clinical questions are answered. A comprehensive treatment of cardiovascular MRI is provided by the textbook by Bogaert and colleagues.3 CT is now well established for the assessment of the thoracic vasculature and large and small airways. Recent advances in multidetector CT (MDCT) have resulted in significant advances in spatial and temporal resolution. This has facilitated coronary artery imaging and gated cine imaging for ventricular function. The rapid acquisition times for MDCT have made it an attractive imaging investigation for CHD. Data can be acquired in a single breath-hold, which in younger patients may obviate the need for general anaesthesia. There are two main disadvantages of MDCT compared with CMR imaging. The first is the use of ionising radiation. This can be kept to a minimum, by using low kV, low mA acquisitions, with current modulation, and image acquisition over the minimal area of interest. Using such protocols, our mean dose for non-cardiac-gated cardiovascular MDCT in children is 1.2 ± 0.57 mSv. This equates to approximately 60 chest radiographs (standard PA chest radiograph = 0.02 mSv) or 6 months of background radiation exposure (UK average background radiation = 2.2 mSv per year). Furthermore, in critically ill patients the ability to perform a rapid examination without the need for general anaesthesia or even sedation may be more crucial, as the risk of prolonged sedation may be greater than that of radiation. The second disadvantage of current MDCT techniques is that easy quantification of cardiac function (at high heart rates) and arterial flow are not possible, especially when compared with cardiac-gated MR imaging. In our own practice, we currently use MDCT for the following indications in patients with CHD: Although CHD may be suspected on the basis of the chest X-ray (CXR), the technique precludes the detailed morphological assessment necessary for diagnosis and determination of specific underlying pathology should be de-emphasised. The diagnostic accuracy of CXR in the assessment of infants with asymptomatic murmurs is poor; combined data from studies indicates the sensitivity and specificity are 0.29 (CI 0.24–0.35) and 0.86 (CI 0.83–0.88), respectively.4,5 Even amongst experienced specialist paediatric radiologists the sensitivity is only 0.30 and amongst radiology trainees it is 0.16. Despite its poor performance as a screening tool, CHD may be suspected on the basis of a CXR due to higher specificity; but false-positive rates are significant. The CXR, however, is not dispensable, and remains important in the subsequent management of patients with CHD, particularly in three situations: 1. Postoperatively, for identification of the position of intravascular catheters, chest drains and endotracheal tubes (Fig. 20-1A); 2. Identification of postoperative complications: consolidation, collapse, pleural effusion, pneumothorax, pneumomediastinum or pericardial collections (Fig. 20-1B); and 3. Perioperative, physiological assessment of the lungs and cardiomediastinal contour (see below). The ubiquity of the CXR in clinical practice warrants discussion of the diagnostic features that should prompt suspicion of CHD. It is suggested that the reader avoid such terms as ‘boot-shaped’ or ‘snowman’ typically associated with specific lesions, when reporting images, as they can be misleading and often erroneous. More appropriate is a descriptive consideration of the cardiomediastinal contour and lungs, attempting to evaluate the predominant physiological profile discussed above. The reader may find it helpful to read this section in conjunction with the section on clinical presentation. Radiologically normal pulmonary vascularity is present in CHD if the patient is not in heart failure, if no large shunt is present, and if there is not a significant reduction in pulmonary blood flow, e.g. mild pulmonary stenosis. The pulmonary vasculature may, however, look normal on the conventional radiograph even in the presence of significant CHD. Increased pulmonary perfusion (pulmonary plethora) is recognised by enlarged central and peripheral pulmonary arteries and veins in all zones (Fig. 20-2A), as occurs in situations with increased pulmonary blood flow: ASD, VSD and PDA with large left-to-right shunts (Table 20-1). Decreased pulmonary perfusion (oligaemia) (Fig. 20-3) is due to a reduction in pulmonary blood flow and is typically a phenomenon of cyanotic CHD. Dark lungs and sparse pulmonary vascular markings suggest the diagnosis. Image acquisition must be optimal, as overexposure will significantly confound correct interpretation. Pulmonary blood flow may be impaired by obstruction to normal flow through the right heart, e.g. tricuspid atresia, tetralogy of Fallot, pulmonary stenosis (Table 20-2). Pulmonary venous congestion and oedema (Fig. 20-2B) in CHD is due to functional or anatomical obstruction to pulmonary venous return. In addition to oedema formation due to increased transvascular pressure gradients, consideration should be given to other pathological processes such as increased vessel leakiness, for example due to acute lung injuries (Table 20-3). The usual adult pattern of basal oedema, resulting in alveolar hypoxia and constriction of lower pulmonary vasculature and redirection to the apices, does not apply to the supine infant. As pulmonary venous pressure increases, there is progressive accumulation of radiological signs, beginning with redistribution (in older children/adults), progressing to interstitial oedema (perivascular haziness, peribronchial cuffing, Kerley B lines, subpleural effusions) and finally migration of extravasated fluid centrally resulting in perihilar alveolar consolidation.
Congenital Heart Disease
General Principles and Imaging
Introduction
Clinical Presentation
The following three physiological states predominantly account for patients with cyanosis:
Later Clinical Presentation
Morphological Description and Sequential Segmental Analysis
Sequential Segmental Analysis
Step 1—Atrial Situs
Step 2—Ventricular Morphology
Step 3—Ventriculo-arterial Connection
Step 4—Identification of Other Abnormalities
Physiological and Functional Assessment
Non-Invasive Imaging Techniques
Ultrasound
Magnetic Resonance Imaging
Computed Tomography
Conventional Radiology
Diagnostic Features
The Pulmonary Vasculature
Radiologically Normal Pulmonary Vascularity.
Increased Pulmonary Perfusion (Pulmonary Plethora).
Decreased Pulmonary Perfusion (Oligaemia).
Pulmonary Venous Congestion and Oedema.
Systemic-to-Pulmonary Collateral Vessels.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree