Abstract
A large range of imaging techniques is now accessible to answer clinical questions raised by CHD patients. This chapter will review the strengths and limitations of each imaging technique and specific indications. In the second part of this chapter, specific CHDs will be described, and the contributions of specific imaging techniques will be discussed.
Keywords
Congenital heart disease, Echocardiography, Cardiac magnetic resonance, Computed tomography
Abbreviations and acronyms
3D
three-dimensional
4 CV
apical 4-chamber view
AICD
automatic implantable cardiac defibrillator
ASD
atrial septal defect
ccTGA
congenitally corrected transposition of the great arteries
CHD
congenital heart disease
CoA
coarctation of the aorta
CMR
cardiac magnetic resonance
CT
computed tomography
LV
left ventricle
LVOT
left ventricular outflow tract
RV
right ventricle
RVOT
right ventricular outflow tract
SPECT
single photon emission computed tomography
TAPSE
tricuspid annular plane systolic excursion
TDI
tissue Doppler imaging
TGA
transposition of the great arteries
TEE
transesophageal echocardiography
TOF
tetralogy of Fallot
TTE
transthoracic echocardiography
VSD
ventricular septal defect
19.1
Introduction
The past 30 years have seen tremendous improvements in diagnosis and treatment of congenital heart disease (CHD). This allows more than 90% of babies born with heart malformation today to reach adulthood. This new population of adults who have CHD brings new problems and diagnostic challenges. Apart from the small number of adult patients who have previously undiagnosed CHD, it is important to recognize surgical corrections, residual lesions, and new complications in previously treated patients. In this setting, cardiac imaging plays an important role in the understanding of cardiac anatomy and physiology, helps to ensure a correct diagnosis of the lesion, and sometimes plays a role in treatment. Recent years have seen a shift from diagnosis by cardiac catheterization to echocardiography and more recently to tomographic techniques such as cardiac magnetic resonance and computed tomography (CT) [ ]. In the first part of this chapter, we will review the different imaging modalities available, their advantages and limitations, and then, in the second part of this chapter, we will analyze the role of the different imaging modalities in specific lesions.
19.2
Imaging modalities
Several imaging modalities are currently available to assess patients with CHD. Cardiac catheterization was for a long time the reference technique for diagnosis.
19.2.1
Echocardiography
Echocardiography is and remains the first-line technique in patients who have CHD. It must be performed in every patient who has or is suspected to have CHD. It has the advantage of being a widely available, low cost, portable, and radiation-free technique, and it provides information on both anatomy and physiology (see Chapter 2 ). CHD may cover a very wide range of lesions, from a simple “hole” such as an atrial septal defect (ASD) or a ventricular septal defect (VSD) to very complex anatomy such as transposition of the great arteries (TGA) corrected by atrial switch. Because the initial anatomy may have been modified by surgical intervention (baffle, conduit, and so on), it is important to precisely know not only the malformation but also all the surgical interventions that have been performed. Therefore, it is important to use a segmental approach when performing an echo on a patient with CHD [ , ]. The first step is to know the position of the heart ( dextro or levo position). This step is best achieved using the subcostal view, which also allows for assessment of the position of the liver. The second step is to recognize the cardiac chambers and their relative positions. For example, the morphological right ventricle has coarse trabeculations and a moderator band, and the tricuspid valve is the only atrioventricular valve with three leaflets and septal attachments (the mitral valve is a two-leaflet valve with attachments on papillary muscles normally located in the anterolateral or posteromedial position). The third step is to look at the arrangement of the great vessels: the aorta and the pulmonary artery. Finally, surgical “modifications” will be investigated.
In addition to this important anatomical knowledge, echocardiography allows assessment of ventricular function and volume or pressure overload. Doppler techniques play a determinant role in the assessment of intracardiac shunts (ASD, VSD, and so on), valvular function (regurgitation or stenosis), right ventricular or left ventricular outflow tract obstruction, surgical conduit or baffle obstruction, and pulmonary pressure using the velocity of tricuspid regurgitant jet.
Despite numerous strengths, echocardiography has some limitations due to poor acoustic windows after surgery or due to unusual heart positions. Some structures, including the pulmonary veins, the great arteries, and the pulmonary artery branches, are difficult to assess. Because of the heart’s particular structure, right ventricular function and volumes may be poorly assessed by echocardiography, especially in the case of a systemic ventricle or tetralogy of fallot (TOF) [ ]. This is also the case for univentricular hearts. Doppler measurements also have limitations and may lead to misinterpretation of the severity of lesions: This is particularly true in aortic coarctation, right ventricular outflow tact obstruction, and serial stenosis [ ].
In some cases, transesophageal echocardiography (TEE) may provide additional information. The main indications accepted for TEE are [ , ]:
- 1.
Diagnostic indications:
- ●
inconclusive transthoracic echocardiography (TTE) in suspected or known CHD (poor TTE acoustic windows)
- ●
evaluation of intracardiac or extracardiac baffles following Fontan or atrial switch repair operation
- ●
- 2.
General indications: infective endocarditis, prosthetic valve function, before cardioversion
- 3.
Perioperative indication
- 4.
For percutaneous procedure guidance: e.g., ASD closure
Tissue Doppler imaging (TDI) was proposed to assess right ventricular function and circumvent one of the limitations of conventional 2D echocardiography [ ]. Because of all the limitations related to the technique, it was never widely used.
Real-time three-dimensional (3D) echocardiography could also provide useful information. In patients who have heart defects (e.g., ASDs), it allows for precise location and measurement of the defect’s dimensions to guide therapeutic decisions. In complex CHD, 3D echocardiography shows the relative position of each structure. It also represents a valuable tool for assessing RV and LV ventricular function and valve disease [ , ].
Deformation imaging, one of the most recent developments in the field of echocardiography, was also used to assess CHD patients. It seems particularly useful to assess ventricular function and detect subclinical impairment [ , ]. In the future, use of 3D speckle tracking echocardiography will certainly open new opportunities to understand ventricular mechanics in CHD [ ].
Stress echocardiography has little indication in CHD except to detect ischemia in coronary abnormalities. Only a few studies have proposed the use of stress echo to assess right ventricular function after atrial switch surgery [ ].
19.2.2
Cardiac magnetic resonance imaging
In the past decade, cardiac magnetic resonance imaging (CMR) has gained a particular place in CHD imaging. This technique has several advantages over echocardiography, including the ability to use unrestricted image planes to assess the different cardiac structures or vessels, and the ability to perform tissue characterization without exposing the patient to any radiation, which is particularly relevant for young patients who may require multiple investigations during their lifetimes. CMR allows evaluation of anatomy of native or post-surgery CHD, in particular ventricular function (right and left), measurement of systemic or pulmonary blood flow, quantification of valvular regurgitation, and identification of myocardial fibrosis.
When using echocardiography, it is important to have a good understanding of the underlying CHD and of the previous surgery or surgeries to obtain the most clinically useful information from CMR imaging. A standard approach should be applied to CHD patients to ensure reproducibility and accuracy of measurements [ ]. Recently, the European Society of Cardiology published standardized CMR protocols to be used for CHD. Most of the studies realized were performed with 1.5 Tesla CMR; today 3.0 Tesla magnets are used more and more frequently. This may permit not only a shorter examination time but also better spatial and temporal definition of the acquired images [ ].
A typical CMR approach to a patient who has CHD is:
- ●
Morphological ( T1-weighted ) imaging typically generates a stack of axial images, which provides an anatomical overview.
- ●
Steady-state free precession imaging allows for anatomical assessment and evaluation of right and left ventricular volume and function. Two-dimensional stacks of cine images will be acquired in several orientations, allowing visualization of different cardiac structures:
- ●
vertical long axis, horizontal, and left ventricular short-axis planes,
- ●
4-chamber and 3-chamber planes,
- ●
a coronal, LVOT plane orthogonal to the first, aligned with the flow through the LVOT and aortic valve,
- ●
aortic arch cine aligned with the arch and proximal descending aorta,
- ●
RVOT cine(s) aligned with the RVOT and proximal MPA,
- ●
a contiguous stack of transaxial cines, from the lower heart border up to the aortic arch,
- ●
a contiguous stack of straight coronal cine images, from the front of the heart to the descending aorta,
- ●
a stack centered on a particular structure, according to the clinical situation; for example, the atrium, mitral valve, aortic valve.
- ●
- ●
Cine images with tissue tagging may be acquired to permit assessment of regional deformation or strain of the myocardium [ ].
- ●
Phase contrast sequences are used to assess flow dynamics. Velocity-encoded images allow measurement of the through phase flow in the aortic and pulmonary arteries, permitting calculation of valve regurgitant volume and fraction and shunt quantification (flow estimate through the pulmonary and the aortic valves). A pitfall of the technique is aliasing; to avoid it, the velocity of encoding should be slightly above the expected peak velocity and increased if necessary. Also, there may be some phase offset that, to be minimized, requires centering the patient in the magnet or a phantom correction [ ].
- ●
Contrast-enhanced magnetic resonance angiography (CEMRA) is used in CHD to visualize anomalous pulmonary or systemic venous return, and aortic pathology, such as coarctation and associated collaterals.
- ●
Late gadolinium – enhanced CMR may be used to view a suspected infarction, infiltration, or scarring [ ].
The main indications for using CMR in CHD include:
- ●
as an adjunct to echocardiography, when additional information is necessary to fully assess the clinical problem;
- ●
to confirm echocardiographic findings in valvular disease, ventricular function and volume, and shunt;
- ●
as a first line tool for assessment of RV function and volumes and aortic coarctation.
Particular challenges of using CMR in CHD include the need for sedation or anesthesia in infants, the faster heart rate in children, occasional claustrophobia, and non-MR compatible implants.
19.2.3
Computed tomography
With the arrival of multidetector systems, CT has improved in performance (shortened acquisition times) and precision (spatial resolution) and is now a well-established tool used to analyze cardiac and pulmonary structures [ ] (see Chapter 5 ). CT is the preferred technique to noninvasively assess the coronary artery anatomy, and it is also well suited to analyze prosthetic valves and large stents (e.g., after treatment of aortic coarctation) [ ]. Additionally, the entire aorta and pulmonary artery can easily be investigated using CT.
ECG-gated CT can be used to obtain volume datasets and calculate left and right ventricular volume and ejection fraction and thus represents an alternative to echocardiography and CMR [ ]. Due to the high spatial and temporal resolution of the latest-generation scanners, CT can analyze intracardiac structures, such as valves, like echocardiography or CMR [ ]. However, the significant radiation exposure patients receive from CT is an important deterrent for wider use of the technique as a first-line test or for repeat imaging studies, particularly in younger patients.
Most CT examinations require injection of a contrast iodine agent. This is contraindicated in cases of allergy to iodine products, hyperthyroidism, and renal failure.
In addition to cardiac and great vessel anatomy, CT gives also information regarding tracheobronchial anatomy, lung parenchyma, and thoracic bone structures, which may be of interest for particular congenital conditions. Use of radiation represents a major drawback of CT, especially when multiple follow-up studies are contemplated or for younger people. Recently, new acquisition protocols have been developed to minimize the dose of radiation, which will potentially help to promote the use of CT in younger patients who have CHD.
19.2.4
Chest X-ray
Chest X-ray can be used for longitudinal assessment of heart size and could be useful for the assessment of pulmonary vascularization. Nevertheless, its routine use is no longer recommended [ ].
19.2.5
Radionuclide imaging
Right or left ventricular ejection fraction and volumes can be calculated by radionuclide angiography using multiple gated acquisitions (MUGAs) obtained after equilibration of 99m Tc-labeled imaging agents. This technique is no longer considered to be a first-line technique because of the lack of other information conveyed about cardiac structure and function and the use of radiation. It must be considered a valuable alternative to assess ventricular function and volume when echocardiography cannot answer the question and when CMR is contraindicated.
In some rare cases of coronary abnormalities or coronary reimplantation, myocardial ischemia could be identified by stress single photon emission computed tomography (SPECT) (see Chapter 3 ).
Lung perfusion scintigraphy using 99m Tc-macroaggregated albumin may be used to determine perfusion in each lung, which could help to determine the physiological repercussions of pulmonary artery or vein stenosis.
19.2.6
Cardiac catheterization
Developments in echocardiography and tomographic techniques have contributed to the drastic decrease in the use of cardiac catheterization for diagnostic purposes. This technique is currently used to solve specific questions that are not answered by other noninvasive techniques or for hemodynamic assessment rather than for imaging. The remaining indications are measurement of pulmonary vascular resistance and reversibility of pulmonary hypertension; and assessment of coronary arteries, collateral vessels, the pulmonary artery, and, in some cases, surgical shunts (e.g., Glenn shunt, Fontan).
Invasive measurement of systolic and end-diastolic ventricular function, pressure gradients, or shunts should be proposed for patients in whom noninvasive evaluation cannot solve the clinical problem, especially in very complex cases of CHD when the information is required for clinical decision making.
In shunt lesions, if pulmonary hypertension is suspected after echocardiography, confirmation of elevated pulmonary pressure and resistance and vasoreactivity testing using oxygen or nitric oxide are important before making the decision to close the shunt.
However, today, the main use of cardiac catheterization in CHD is for an interventional procedure.
19.2.7
When to use specific imaging
As outlined above, a wide range of imaging techniques is available to assess CHD, often with overlapping indications. Nevertheless, particular techniques are more suited to answer specific questions. Recently, Orwat et al. presented a proposal to appropriately use the different imaging modalities based on the recommendations of the European Society of Cardiology [ , , ]. An adapted version is listed here:
- ●
Echocardiography is preferred as the first imaging modality to evaluate a patient who has CHD and to try to solve the clinical problem.
- ●
Echocardiography is superior to other imaging techniques for assessment of vegetation or small mobile structures.
- ●
CMR or CT can be used as an alternative technique to echocardiography if reliable information to make a clinical decision cannot be obtained by echocardiography due to poor echocardiographic image quality.
- ●
CMR can be used a second choice when echocardiographic measurements are borderline or need to be confirmed, for example, for ventricular function and volumes (regurgitant and shunt lesion) or assessment of the severity of shunt or regurgitant lesions.
- ●
For some indications, CMR or CT is considered to be superior to echocardiography and bring more valuable information for management of CHD patients. These are:
- ●
quantification of RV volumes and ejection fraction (TOF, systemic RV, and tricuspid regurgitation) (CMR),
- ●
evaluation of the RV outflow tract and RV-PA conduits (CMR and CT),
- ●
quantification of pulmonary regurgitation (PR) (CMR),
- ●
evaluation of pulmonary arteries (stenoses and aneurysms) and the entire aorta (aneurysm, dissection, coarctation) (CMR and CT) and stents (CT),
- ●
evaluation of systemic and pulmonary veins (anomalous connection, obstruction, and so on) (CMR and CT),
- ●
collaterals and arteriovenous malformations (CT is preferred over CMR),
- ●
coronary anomalies and coronary artery disease (CT is preferred over CMR),
- ●
evaluation of intra- and extracardiac masses (CMR and CT),
- ●
quantification of myocardial masses (CMR and CT),
- ●
detection and quantification of myocardial fibrosis/scar (gadolinium late enhancement) (CMR),
- ●
tissue characterization (fibrosis, fat, iron loading, and so on) (CMR).
- ●
When both CMR and CT could be used, the choice between these techniques must take into account the local expertise, the presence of any allergy to the iodine component, the repeated use of radiation, and the presence of a pacemaker or automatic implantable cardioverter-defibrillator (AICD).
19.3
Specific lesions
19.3.1
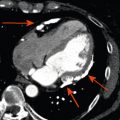
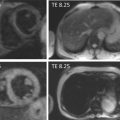
Atrial septal defect
ASD is the most frequently encountered CHD that goes undiagnosed until adulthood. Indeed, ASD can remain asymptomatic until an advanced age. Several types of ASD can be differentiated:
- ●
The ASD ostium secundum is the most frequent form of ASD, representing 80% of the lesions. It is located in the region of the fossa ovalis, and it is the most frequent form diagnosed in adults.
- ●
The ASD ostium primum is located near the crux of the heart and accounts for approximately 15% of ASDs. It is frequently associated with mitral or tricuspid valve malformation or with VSD, forming a partial or complete atrioventricular canal. Most of the time it will be cured during childhood.
- ●
The sinus venosus ASD is located at the mouths of the caval veins (inferior and superior vena cava) and is frequently associated with partial abnormal venous return.
- ●
Unroofed coronary sinus is a very rare form of ASD characterized by a communication between the coronary sinus and the left atrium. It is associated with a persistent left superior caval vein.
Imaging plays a crucial role in the management of ASD. First, imaging confirms the diagnosis of ASD and its location. Second, imaging is used to quantify the severity of the interatrial shunt and the repercussions of the ASD (e.g., RV dilatation, pulmonary hypertension). Finally, if percutaneous closure is considered, imaging confirms the feasibility and guides the procedure.
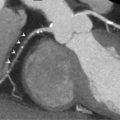
Echocardiography is the key examination in a case of suspected ASD. TTE examination must assess the interatrial septum from the parasternal modified long axis, short axis, apical, and subcostal views ( Figure 19.1 ). Color Doppler is used to confirm the shunting. The RV size and volume overload will also be determined using the same views. When an unexplained RV enlargement is discovered, an atrial shunting should always be examined. The severity of the shunt is calculated by the ratio of blood flow in the systemic and pulmonary circulation (Qp/Qs). The calculation requires the measurement of the diameter of the RVOT and LVOT, and the time velocity integral of pulmonary and aortic flow obtained by pulsed Doppler. Unfortunately, measurement is hampered by several potential errors, mainly in the RVOT measurement. Therefore, the clinical use is limited, and international recommendations rely more on RV dilatation to prove the hemodynamic burden of ASD [ , ].


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree