Anomalies of SVC
Persistent left SVC (connection to coronary sinus)
Persistent left SVC with unroofed coronary sinus
Persistent left SVC connecting to left atrium
Absent right SVC with persistent left SVC
Right SVC connecting to left atrium
Aberrant right atrial insertion
Aneurysm
Absent both SVC with azygos continuation of IVC
Anomalies of IVC
Azygos continuation to SVC
Hemiazygos continuation to left SVC
Connection to left atrium
Connection to coronary sinus
Aberrant right atrial insertion
Situs inversus
Miscellaneous thoracic venous disorders
Absent left brachiocephalic vein with venous return via (LSICV)
Anomalous courses (subaortic) of the left brachiocephalic vein
Anomalous hepatic venous connection to right atrium
Anomalous hepatic venous connection to coronary sinus
Absent azygos vein
Common associated anomalies
Heterotaxy syndrome, TOF
Table 26.2
Classification of pulmonary venous anomalies
Anatomical variant in connection or course: common trunk, supernumerary vein, meandering pulmonary vein |
Intrinsic abnormality: stenosis, hypoplasia, atresia, aneurysm (varix) |
Partial anomalous pulmonary venous return |
Right pulmonary veins: SVC, right atrium, both, IVC (scimitar), azygos vein |
Left pulmonary veins: left brachiocephalic vein (left vertical vein), coronary sinus |
Partial bilateral |
Total anomalous pulmonary venous return |
Supracardiac: left brachiocephalic vein, right SVC, azygos vein |
Cardiac: coronary sinus, right atrium |
Infracardiac: portal vein or tributary, ductus venosus, IVC, left gastric vein |
Associated anomalies |
Pulmonary: bronchopulmonary sequestration, AVM, congenital cystic adenomatoid malformation |
Common cardiac: cor triatriatum |
Imaging Techniques
Thoracic venous anomalies may not be diagnosed until adulthood. The decision to perform surgical correction relies on preoperative knowledge of the systemic or pulmonary venous anatomy, associated cardiac anomalies, and the magnitude of the left-to-right or right-to-left shunts. Multiple complementary diagnostic tests are usually necessary in order to obtain complete anatomic and functional data. Although echocardiography has a variety of diagnostic strengths especially in the evaluation of many congenital heart disease in children, it can be suboptimal for comprehensive assessment of thoracic venous anomalies. With magnetic resonance imaging (MRI) or computed tomography (CT), detailed anatomic information regarding the number, origin, course, and drainage of all thoracic veins and their relationships to cardiac and extracardiac structures can be easily obtained. They provide volumetric data that are essential for diagnosis and therapeutic planning [7–11]. Associated great vessels and intracardiac anomalies can also be evaluated. Both modalities are useful postoperatively and facilitate the evaluation of patency, anastomotic narrowing, right ventricular systolic function, and residual abnormal communications.
CT provides rapid acquisition of data with high spatial resolution and a wide anatomic coverage. Isotropic multiplanar reformatting and volume rendering reconstruction capabilities both excellently depict anomalous pulmonary venous structures [7]. The primary disadvantage of CT is that it requires the use of ionizing radiation. Using high pitch modes and iterative reconstruction methods radiation dose can significantly be reduced. Major advantages of MRI are the lack of ionizing radiation and the power for dynamic imaging and function analysis with or without contrast. However, when compared with CT angiography, high-quality MRI capabilities are not nearly as widely available and currently do not offer a comparable level of spatial resolution, and susceptibility artifacts can be problematic. In patients with a cardiac pacemaker or claustrophobia, CT may be a better choice.
In both CT and MR angiographies, timing of the intravenous contrast material bolus is critical because a suboptimally timed bolus may limit opacification of targeted venous structures. In a patient with left-sided SVC, a left arm approach may be the preferred route of injection. In postoperative patients with bidirectional cavopulmonary shunts and azygos continuation of IVC, it may be necessary to perform a dual injection method using both arms or one arm and one lower extremity injections simultaneously.
Regarding CT technique, the narrowest allowable beam collimation should be used to produce isotropic reformatted and 3-dimensional volume-rendered images. Most scanners have the capability of 0.5 mm collimation that can easily provide an isotropic resolution of 0.5 mm in a 25-cm field of view. Nonionic low-osmolality (or iso-osmolality) iodinated contrast material (1–1.5 mL/kg, 80–120 mL volume) is typically administered intravenously using a power injector at injection rates of 3–5 mL/s, depending on quality of venous access. In the evaluation of systemic venous anatomy, diluted contrast (30–50 %) without bolus triggering is the preferred method of injection. The scan can be started immediately after contrast injection or with a few seconds delay depending on “scan start delay” timing specific to the machine used. Bolus triggering generally delays scan initiation for an additional 5–7 s. Without triggering this can be reduced to 2–3 s delay. For pulmonary vein anatomy, anomalous pulmonary venous drainage, and cor triatriatum, the timing of imaging after the injection of contrast material may be precisely determined using either a timing bolus or real-time contrast material tracking. In general the CT technique for evaluation of the pulmonary veins is very similar to coronary CT angiography. In our institution we prefer to start scan with contrast arrival in the descending thoracic aorta below the level of tracheal bifurcation using a 180 Hounsfield unit trigger threshold. Generally, cardiac gating is not required for the evaluation of systemic or pulmonary venous structures, although it may prove useful if the patient is being specifically evaluated for cor triatriatum, associated intracardiac lesions, or central pulmonary vein hypoplasia/stenosis.
MRI is the preferred imaging technique for noncontrast imaging of venous system, flow quantification, and functional analysis [11–13]. Not only can MRI detect the extracardiac anomalous vessels, it can also clearly delineate the intracardiac anatomy (i.e., sinus venosus defect). Furthermore, velocity flow mapping allows accurate assessment of the Qp/Qs without the need for or the risk associated with cardiac catheterization. The thoracic venous system can be evaluated using a variety of MRI techniques. Images are ideally acquired using a dedicated phased-array cardiac coil. Both black blood and bright blood images may be obtained without intravenous contrast material. High-resolution double-inversion recovery fast spin-echo (black blood) images are typically acquired in axial, coronal, and sagittal directions to assess anatomy. Fast gradient echo and balanced steady-state free precession (b-SSFP) pulse sequences provide bright blood cine images that are useful for the venous stenosis, thrombosis, or anomalies. These sequences can be applied using 2-dimensional (2D) or 3D modes without and with ECG gating. The use of contrast-enhanced MR angiography in patients with anomalous thoracic veins has been advocated as a way to improve diagnosis and reduce the patient’s time in the scanner. However, recent concerns about complications associated with gadolinium contrast agents have led to using other imaging techniques that do not require the administration of gadolinium contrast. Noncontrast respiratory-triggered ECG-gated free-breathing T2-prepped 3D b-SSFP pulse sequences allow the acquisition of a near-isotropic volumetric dataset (1.5 mm3) that can be reformatted and reviewed in any plane [14]. For respiratory triggering a navigator pulse with a 5-mm acceptance window is placed over the right hemidiaphragm. A large (40 cm) field of view permits imaging of the entire chest. To reduce the sensitivity of SSFP to field inhomogeneities, which can be problematic for large field-of-view imaging, a nonselective radio frequency excitation is used to shorten the repetition time. Unenhanced MR angiography using this technique is shown to be as accurate as contrast-enhanced MR angiography for showing pulmonary veins [14]. The SSFP technique also permits evaluation of the extravascular pathologies.
For contrast-enhanced MR angiography, a non-ECG-gated 3D fast gradient is usually used [15, 16]. Gadolinium-based contrast material may be either injected by hand or power-injected at 3–4 mL/s using a double dose (0.20 mmol/kg). Depending on the desired anatomical coverage, large field-of-view 1.5–2 mm oblique coronal or sagittal images (i.e., imaging slab of 60–80 mm are partitioned into 40 slices) can be acquired using two or three sequential breath holds or during quiet breathing (Fig. 26.1). A delay of 5 s can be used for the systemic vein evaluation and 15–20 s for the pulmonary veins. Newer dynamic, time-resolved 3D MRA sequences using high-performance gradients obviate the need for a timing strategy. Because a complete 3D MRA dataset can be acquired in 4–7 s, several sequential acquisitions can be obtained immediately following contrast injection. Subsequently, the best quality dataset is selected for image analysis. Although reviewing the individual source images is important, these images are typically reformatted in multiple planes as well as reconstructed as either maximum-intensity-projection (MIP) or volume-rendered images [17].
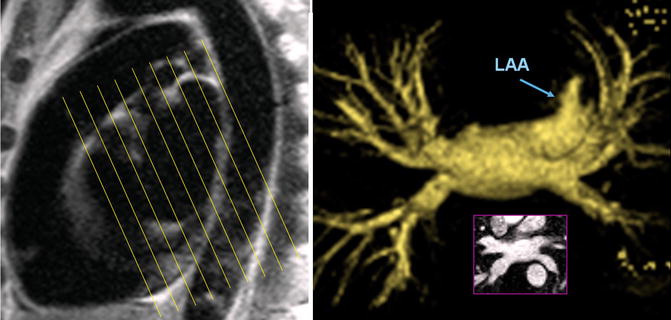

Fig. 26.1
MR angiography technique for the left atrium and pulmonary veins. Using a sagittal reference, oblique coronal partitions will be obtained parallel to the short axis of the heart. An anteroposterior angle (approximately 20°) brings the plane of 3-dimensional dataset parallel to the long axis the pulmonary veins. LAA left atrial appendage
In addition to anatomy, MRI can be used to detect and measure shunts in a patient with anomalous venous return [13, 18, 19]. Classically the oximetry with cardiac catheterization remains the most widely accepted clinical standard for the QP/QS measurement. However, very small shunts, especially at the atrial level, are hardly assessed by the currently used oximetric technique, because of the pO2 right chamber variability observed even in normal subjects without left-to-right shunt [20]. On the other side the accuracy of MRI QP/QS assessment in vitro and in vivo has already been demonstrated [18]. Phase-contrast MRI flow measurements have been shown more reproducible and tended to correlate better with defect area than oximetry calculations.
Systemic Venous Anomalies
Embryology
Systemic venous anomalies of the thorax are the result of complex variations in the persistence and regression of segments of three pairs of venous channels during the first 4 weeks of fetal development. All these three venous channels drain into the sinus venosus: the omphalomesenteric (vitelline) veins, carrying blood from the yolk sac; the umbilical vein, originating in the chorionic villi and carrying oxygenated blood from placenta; and the common cardinal veins (ducts of Cuvier), draining the body of the embryo.
The right and left common cardinal veins are formed by the confluence of the anterior and posterior cardinal veins [1, 21, 22] (Fig. 26.2). The anterior cardinal vein drains the cephalic portion of the embryo, and the posterior cardinal vein drains the remainder of its body. The left vitelline and cardinal veins regress during embryogenesis. The right vitelline vein forms hepatic segment of the IVC (hepatocardiac channel). The right horn of the sinus venosus and the right common cardinal vein will be developed and eventually form the posterior wall of the right atrium (RA) and the SVC, respectively. The left horn of the sinus venosus, in conjunction with the regressing left common cardinal vein, forms the coronary sinus (CS) and the ligament or vein of the left atrium (Marshall) (Fig. 26.2). A bridging venous plexus forms between the right and left anterior cardinal veins around 24 mm crown-rump embryonic stage and develops to be the left brachiocephalic vein [23]. The left anterior cardinal vein below this connection gradually obliterates. Its upper portion remains as part of the left superior intercostal vein (SICV). The right anterior and common cardinal veins persist and form the SVC.
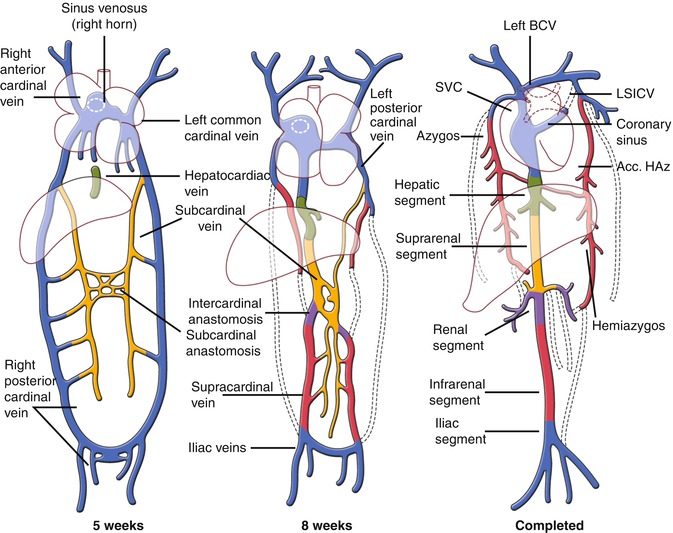

Fig. 26.2
Development of the cardinal veins. The relative relationship and the timing of development of the structures in cartoons are speculative. Anterior views. Acc accessory, BCV brachiocephalic vein, HAZ hemiazygos, LSICV left superior intercostal vein, SVC superior vena cava
The right sinus valve persists as the valve of the IVC (eustachian valve) and the valve of the coronary sinus (thebesian valve) [24]. A double SVC is the result of persistence of the left anterior cardinal vein. If, in addition, the normally persistent right cardinal vein regresses, then there is only one SVC on left. In the normal atrial situs, persistence of the left anterior cardinal vein connected to the left atrium (LA) is an infrequent occurrence, this vein usually draining into the RA via the coronary sinus. In atrial isomerism, conversely, the left anterior cardinal vein usually persists, draining either into the roof of the left-sided atrium (mainly in right atrial isomerism) or into the CS (usually in left atrial isomerism).
Anomalies of the Right SVC
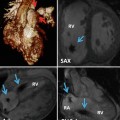
The SVC starts at the confluence of the right and left brachiocephalic veins. It travels 5–7 cm on the right anterior aspect of the upper mediastinum from the level of first sternocostal junction to the superior cavoatrial junction. At the junction with the RA, the SVC is located posterolateral to the ascending aorta, anterior to the right pulmonary artery, and anteromedial to the right superior pulmonary vein (Fig. 26.3). Isolated anomalies of the right SVC are rare [25]. Anomalous low insertion of the right SVC into the right atrium can be seen as an isolated anomaly. Anomalous high insertion of IVC can be seen in congenital heart diseases (CHD). A case of cyanotic CHD with left ventricular hypertrophy described that cardiac catheterization showed a right SVC draining into the left atrium without other cardiovascular abnormality [26]. Congenital aneurysmal dilatation of the SVC may be mistaken with a mass [27] (Fig. 26.4). It may involve the SVC only or multiple veins in the upper mediastinum (Fig. 26.5). This anomaly is usually an incidental finding but has been associated with thrombosis leading to embolization and SVC obstruction [28]. Congenital aneurysmal dilatation can be associated with cystic hygromas [29]. Agenesis of the SVC with two brachiocephalic veins draining separately is also reported without intracardiac malformations [30].
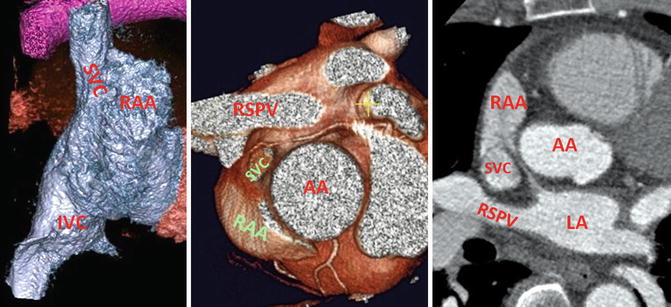
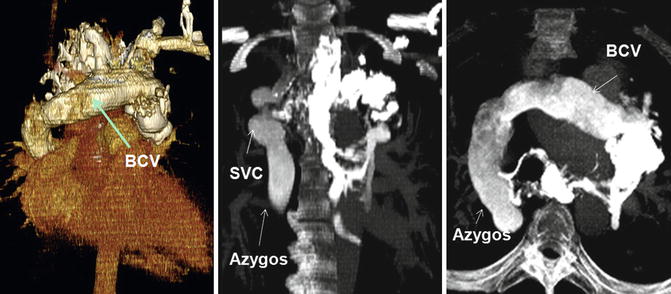
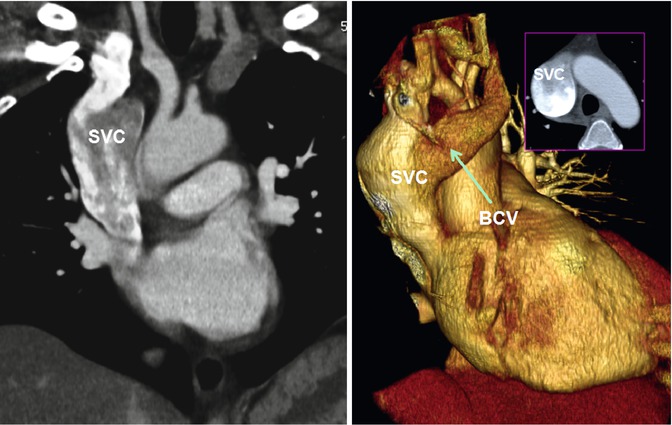

Fig. 26.3
Anatomy of the superior and inferior cavoatrial junctions. Relationship of the superior vena cava (SVC) with the right superior pulmonary vein (RSPV) and the ascending aorta (AA) is shown. Note the SVC connects to posteromedial aspect of the right atrial appendage (RAA). LA left atrium

Fig. 26.4
Varicoid veins of upper mediastinum mainly involving the left brachiocephalic vein (BCV), azygos system, and superior vena cava (SVC) above the confluence with the azygos

Fig. 26.5
Superior vena cava (SVC) aneurysm above the confluence with the azygos vein. The azygos vein was normal sized. The left brachiocephalic vein (BVC) is mildly enlarged. The patient referred to CT for possible mass seen on chest X-ray
Left SVC
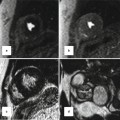
The left SVC is the most common anomalous systemic vein drainage in the thorax, incidentally found in one out of 200 (0.5 %) of CT or MRI studies of chest. In patients with congenital heart malformation, it is more common and may be seen in 5–10 % of patients [1, 31–33]. Isolated left SVC is less common than duplicated SVC. Similar to PAVR of the left upper lobe, it results from failure of parts of the left anterior and common cardinal veins to regress. The left SVC drains the tributaries of the left subclavian and jugular veins into the CS via the oblique vein of the left atrium (vein of Marshall). The LSIV becomes a tributary of the left LVC in 20 % of cases and may connect it to the accessory hemiazygos, analogous to the azygos arch on the right that connects the right SVC to the azygos vein (Fig. 26.6). In 80–90 % of patients, the left and right SVCs coexist, although the left is usually smaller especially when the left brachiocephalic vein bridges the two veins (Fig. 26.7). In about 40–60 % of cases, the left brachiocephalic vein connecting the two SVCs is absent [31, 34]. In this situation the left SVC is larger (Fig. 26.8). In persistent left SVC, especially isolated left SVC, the incidence of CHD increases including atrial septal defect (ASD), tetralogy of Fallot, heterotaxy, coarctation of aorta , pulmonary atresia or stenosis, cor triatriatum, atrioventricular septal defects, double-outlet right ventricle, and anomalous pulmonary venous drainage [1, 35] (Fig. 26.9).
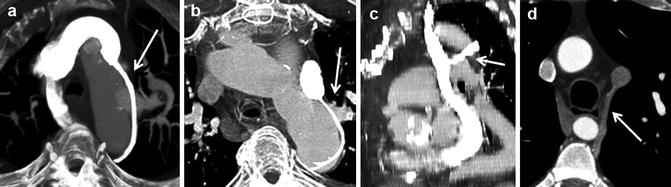
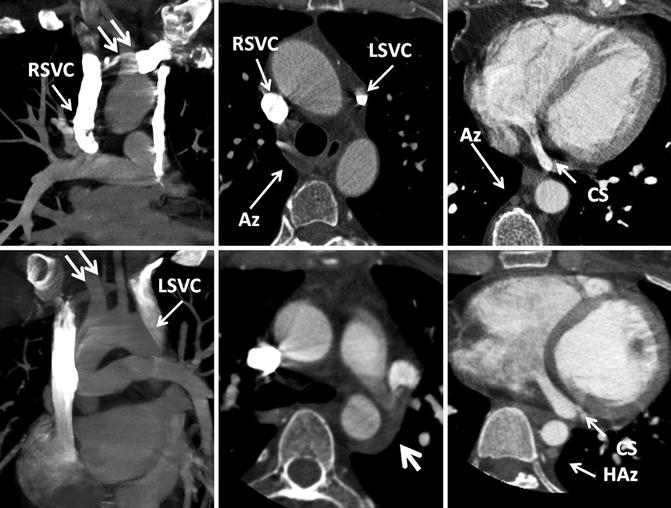
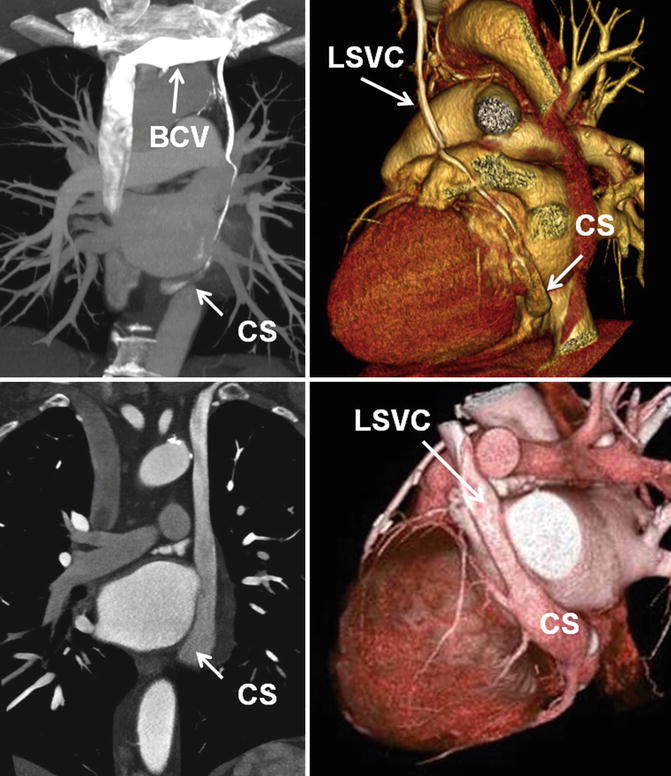
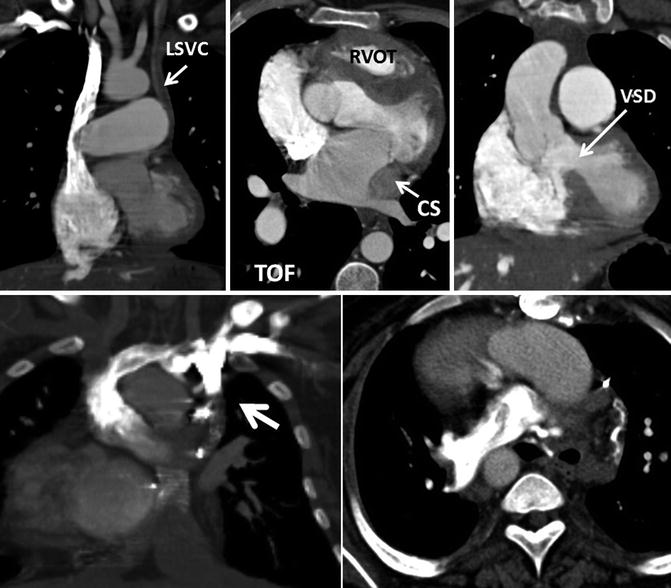

Fig. 26.6
Different patterns of left superior intercostal vein (LSICV) (arrows). (a) Normal with right superior vena cava (SVC) only. (b) Duplicated SVC and small LSICV. (c) Left SVC with large LSICV. (d) Duplicated SVC with LSICV entering left and the azygos entering right SVC. SVC superior vena cava

Fig. 26.7
Double superior vena cava (SVC) system connecting through a normal brachiocephalic vein (double arrow) in two different patients. The left SVC (LSVC) drains into a normal-sized coronary sinus (CS) in both cases. In the first case (upper row), azygos vein (Az) connects to the right SVC (RSVC). In the second case (lower row), the azygos vein is absent but the hemiazygos (HAz) connects to the LSVC by the left superior intercostal vein (large arrow)

Fig. 26.8
Double superior vena cava (SVC) system with (upper row) and without (lower row) left brachiocephalic vein (BCV). Note the coronary sinus (CS) is generally larger in the absence of left brachiocephalic vein. The size of left SVC (LSVC) is variable in the presence of left brachiocephalic vein but it is generally smaller than the right SVC

Fig. 26.9
Upper row: a 27-year-old male with unrepaired tetralogy of Fallot (TOF). Absence of the brachiocephalic vein and a left superior vena cava (LSVC) draining into a large coronary sinus is shown. Note the hypertrophied right ventricle outflow tract (RVOT) and the ventricular septal defect (VSD). The pulmonary arteries are enlarged. Lower row: ligated left superior vena cava (SVC) is shown (arrow) in a patient with dextrocardia, and double-outlet right ventricle, status post bilateral Glenn shunts and Fontan procedure. Injected contrast has entered into the right pulmonary artery through a right cavopulmonary anastomosis
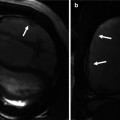
The left SVC descends lateral to the aortic arch and anterior to the hilum, enters the pericardium in the posterior atrioventricular groove, and in almost all cases drains into the CS (90 %) with no significant hemodynamic effects (Figs. 26.8 and 26.10). The CS is large especially when the brachiocephalic vein is absent and will be heavily opacified in CT or MR angiographies when contrast is injected in the left arm. Rarely (8 %), the drainage of the left SVC is not into the CS, but instead into the left atrium or left superior pulmonary vein. In these cases as well, the incidence of CHD increases (Fig. 26.11). Isolated connection of the left SVC to the left atrial roof is very rare [36–38]. Patients with an isolated left SVC draining into the left atrium usually have a right-to-left shunt, unless there is a big bridging vein between the two SVCs, which may allow a left-to-right shunt, instead. Usually, the left SVC draining into the left atrium causes mild cyanosis and polycythemia. Patients are minimally symptomatic or asymptomatic. Cardiac examination, chest X-ray, and electrocardiogram are usually normal. As a result, these rare cases are often an incidental finding during other diagnostic exams [36]. A major complication of this form of hidden right-to-left shunt would be paradoxical embolism with complication such as cryptogenic stroke (Fig. 26.11). Similar situation can occur in cases of left SVC with an unroofed CS especially if the CS ostium to the right atrium is atretic. The anomalies can also interfere with catheterization and may cause difficulty placing a cardiac pacer or defibrillator leads when a left arm approach is used. During the development of the embryo, the left SVC draining into the CS causes an enlargement of this vessel and dissolution of the wall of the CS adjacent to the LA causing either a coronary sinus–left atrium fenestration (unroofing) or an interatrial communication through the mouth of the CS (coronary sinus type ASD) [39]. Therefore, unroofed CS is mostly associated with a left SVC, with or without a connection between both the two SVCs. The diagnosis of this lesion is important to the prognosis of the patient because of the consequences of brain abscess or cerebral emboli that may result from a right-to-left shunt [40, 41]. Injection of contrast in the arm during CT or MR angiography can improve demonstration of this anomaly and associated right-to-left shunting.
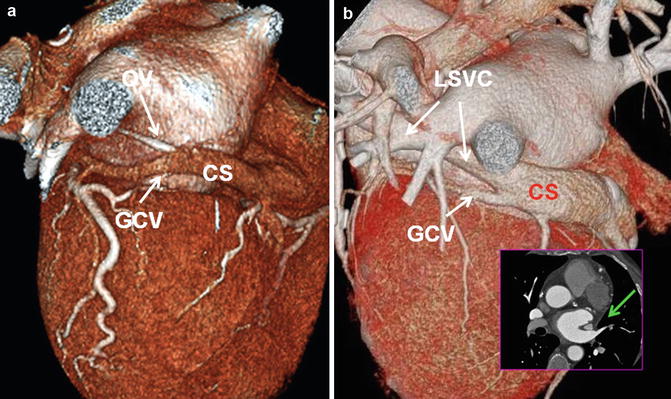
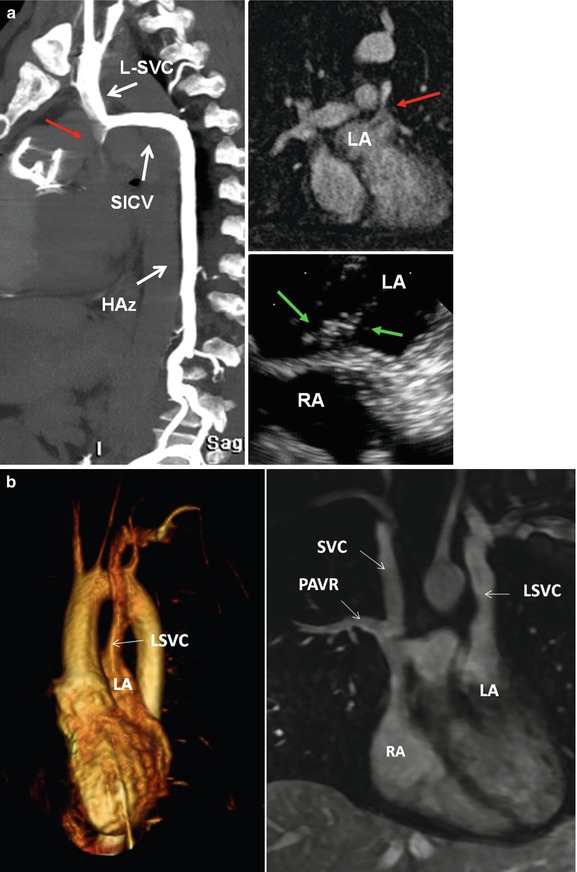

Fig. 26.10
(a) Posterolateral view of the heart demonstrates a normal relationship between the coronary sinus (CS) and the oblique vein of left atrium (OV). The OV is much smaller than the CS and connects to the CS with an angle between 30° t5°. (b) In persistent left superior vena cava (LSVC), the CS is generally larger than normal. The OV and the LSVC pass along the lateral wall of the left atrium between the left atrial appendage and the left superior pulmonary vein (green arrow in b). GCV great cardiac vein

Fig. 26.11
(a) Direct communication of the left superior vena cava (LSVC) with the left atrium (LA) in a patient repaired tetralogy shown by CT and MR angiographies (red arrows) using a left arm injection of contrast method. Maximal intensity projection CT in lateral view (left image) shows that the LSVC is also connecting with the hemiazygos system (HAz) by the superior left intercostal vein (SICV). This was confirmed with transesophageal echocardiography after injection of agitated saline in the left arm. Echocardiography image showing bubbles first entered the LA (green arrows). The communication was coil embolized as the patient had multiple episodes of paradoxical embolic brain infarctions. (b) Volume-rendered left heart MR angiogram from left-sided venous injection in a patient with congenital corrected transposition of the great arteries showing direct communication of the left superior vena cava (LSVC) to the left atrium (LA). There is no connection between the two SVCs. Note partial anomalous venous return (PAVR) of the right upper lobe into the right SVC. RA right atrium
Bilateral SVCs are seen in 60–70 % of patients with situs anomalies [35, 42]. In right atrial isomerism, the left SVC drains into the roof of the left-sided atrium. In the hearts with right atrial isomerism, a coronary sinus defect does not exist as there is no orifice of the CS [39]. Whereas in left atrial isomerism, bilateral SVCs are usually present, and both tend to connect low on the atrial wall near the atrioventricular junction [36]. In 50 % of the cases, the left SVC connects to the right-sided atrium through a CS, but both veins can connect directly into the superior atrial wall.
In situs anomalies, the current trend is to divide and anastomose both SVCs to the pulmonary arteries in a bidirectional cavopulmonary anastomosis, hemi-Fontan, or Fontan procedure [43]. However, a small-caliber left SVC, especially when associated with a large innominate vein, may not be considered worthy of anastomosing and may simply be ligated. In adult repaired CHD the residual of a ligated left SVC can seen in CT or MR (Fig. 26.9). If the left SVC draining the CS is preserved during the Glenn and Fontan-type operations, the ensuing systemic venous hypertension could adversely affect the coronary flow and myocardial perfusion. A persistent left SVC is rarely associated with coronary sinus orifice atresia [44, 45]. Other congenital cardiac lesions associated with coronary sinus orifice atresia with left SVC include ASD, ventricular septal defect, transposition of the great arteries, tricuspid atresia, and mitral atresia [32].
Left Superior Left Intercostal Vein (LSICV) Collateral Pathway
The residuals of an obliterated left anterior cardinal vein are seen as the oblique vein of the left atrium (Marshall) on the cardiac end and as a small vertical vein on the venous end between the LSICV and the left brachiocephalic vein [22, 31, 46] (Fig. 26.10a). The LSICV is a bridging vessel that links the posteriorly located azygos–hemiazygos systems with the anteriorly located left brachiocephalic vein. The LSICV drains the second, third, and fourth posterior intercostal veins and then passes forward and upward along the aortic arch to drain into the left brachiocephalic vein near the venous angle. In 2/3 the vein connects to the accessory hemiazygos vein which is recognizable in most CT angiographies [47, 48] (Fig. 26.6). The LSICV is described as “aortic nipple” in up to 10 % [47] of chest radiographs. Obviously with CT, it can be seen more frequently (45 %) along the lateral side of aortic arch. This bridging venous pathway has the potential of creating a right-to-left shunt when the SVC or left brachiocephalic vein is narrowed or obstructed. In this situation, venous connections develop between mediastinal veins or tributaries of the LSICV on the systemic side and the superior pulmonary veins (rarely left atrium) on the pulmonary side [49].
Left Brachiocephalic Vein
The brachiocephalic veins are formed by the junction of the internal jugular and subclavian veins behind the sternoclavicular joint. The left brachiocephalic vein passes obliquely from left to right, and the right brachiocephalic vein moves downward behind the manubrium. They join to form the SVC. The left vein is longer than the right; neither has a valve. Anomalous brachiocephalic vein is uncommon, accounting for approximately 1 % of congenital cardiovascular anomalies [23, 50]. The cause of an anomalous brachiocephalic vein remains controversial and is related to abnormal regression of the anterior cardinal anastomosis. The presence of two or more (a plexus) transverse precardinal anastomoses is proposed to explain four patterns of anomalous brachiocephalic vein development: anomalous subaortic left brachiocephalic vein, persistent left SVC with a hypoplastic left brachiocephalic vein connecting to the right SVC, double SVC with agenesis of the left brachiocephalic vein [23], and circumaortic left brachiocephalic vein (Figs. 26.12 and 26.13). An anomalous subaortic left brachiocephalic vein is usually associated with congenital heart disease including right-sided aortic arch and underdeveloped pulmonary artery [23, 51]. This vein, rather than joining the right brachiocephalic vein ventral to the aorta, crosses the midline dorsal to the ascending aorta to join the SVC caudal to the azygos vein (Fig. 26.12). Recognition of the brachiocephalic vein anomalies is important to avoid misdiagnosis and prevent technical difficulty in a left arm approach for the insertion of a central venous catheter and cardiovascular intervention. Congenital aneurysm of the brachiocephalic vein is rare [52]. Most aneurysms are asymptomatic and usually manifest incidentally as mediastinal widening on chest radiographs. There may be a marked difference in the appearance of the aneurysm depending on the patient’s posture. A brachiocephalic vein aneurysm can manifest as a mediastinal mass on a supine radiograph but can be barely seen with the patient in the erect position. In this case CT or MR can be diagnostic (Fig. 26.4). A brachiocephalic vein aneurysm, however, can be complicated by a pulmonary embolus, rupture, and venous obstruction, necessitating surgical repair [8].
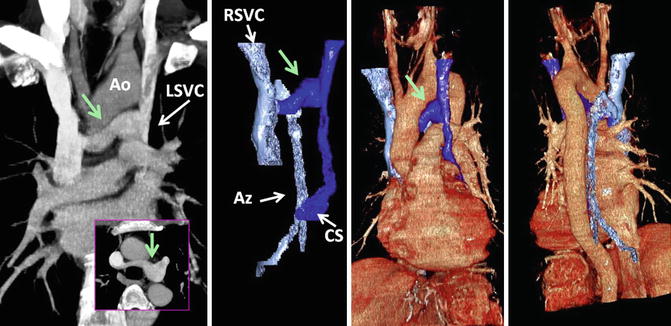
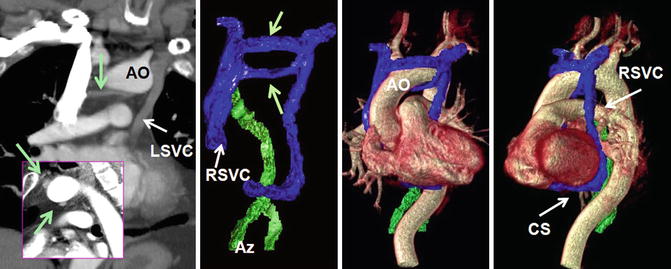

Fig. 26.12
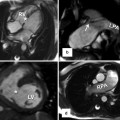
Retroaortic left brachiocephalic vein (green arrow) with persistent left superior vena cava (LSVC). The azygos vein (Az) enters normally into the right SVC (RSVC) and the anomalous brachiocephalic vein connects to SVC caudal to the azygos ostium. Ao aorta, CS coronary sinus

Fig. 26.13
Circumaortic left brachiocephalic vein (green arrows) with dual superior vena cava (SVC) system. The azygos system (Az) connects with the right SVC (RSVC) and the left SVC (LSVC) connects with the coronary sinus (CS), Ao aortic arch
Azygos System and IVC
In the thorax the azygos system receives blood from the posterior intercostal and mediastinal venous tributaries. The superior intercostal vein drains the second through the fourth intercostal spaces. The right superior intercostal vein communicates with the azygos knob. The left SICV drains into the left brachiocephalic vein along the lateral margin of the aortic arch and may connect to the accessory hemiazygos in 70–80 % of cases. The azygos vein drains into the SVC just cephalad to the right main bronchus. The hemiazygos vein crosses midline at T8–T9 level and behind the descending thoracic aorta to join the azygos vein (Fig. 26.14). From this point, the accessory hemiazygos vein extends further cephalad in a left paravertebral position and may communicate with the azygos vein at different levels.
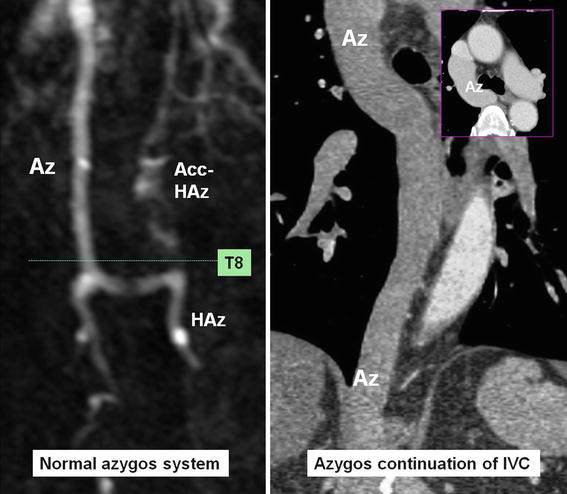

Fig. 26.14
Normal azygos system is shown in MR angiography. The hemiazygos (HAz) and accessory (Acc.) HAz connect each other approximately at T8 level and cross-link with the azygos (Az) at the same level. In azygos continuation of inferior vena cava (IVC), the azygos is markedly enlarged and the hemiazygos system is diminutive
Embryology
During the fourth to sixth weeks of embryologic development, a pair of posterior cardinal veins drain all but the cephalic portion of the embryo [31, 53, 54]. Later at sixth week, a second new pairs of vein, the subcardinal veins, develop which become dominant at 7 weeks while most of the posterior cardinal veins gradually disappear (Fig. 26.2). Important anastomoses develop between the right and left subcardinal veins anterior to the aorta. The right subcardinal vein further develops to the renal and suprarenal portions of the IVC which connect with the posthepatic segment of IVC (derived from vitelline vein). At 8 weeks, the posterior cardinal veins will be replaced proximally by a third pair of veins, the supracardinal veins, which develop medial and dorsal to the posterior cardinal veins and later anatomose each other behind the aorta. The azygos vein derives mainly from the upper right supracardinal vein but the azygos arch seems to originate from the remaining of the cranial segment of the right posterior cardinal vein. Similarly, upper left supracardinal vein turns into the hemiazygos vein, and the residual of the cranial segment of the left posterior cardinal vein may form the posterior portion of LSICV. The IVC development is complex and five embryological segments contribute to its composition. In caudal–cranial order, these segments include posterior cardinal veins (iliac segment), right supracardinal vein (infrarenal segment), anastomosis between the right supra- and subcardinal veins (renal segment), right subcardinal vein (suprarenal segment), and hepatocardiac canal (hepatic segment).
Anomalies
Azygos and Hemiazygos Continuation of the IVC
Abnormal connection of the hepatic and suprarenal segments of the IVC results in azygos or hemiazygos continuation [53, 54] (Fig. 26.14). Therefore, although a large azygos or hemiazygos vein is the predominant finding in this abnormality, the primary anomaly is the IVC maldevelopment.
These anomalies may be isolated or associated with other anomalies (Figs. 26.15 and 26.16). It can be a part of situs anomalies with left isomerism, in which the liver and stomach are located in the midline, multiple spleens are found along the greater curvature of the stomach (polysplenia syndrome), and the minor fissure of the right lung is absent [55]. Azygos continuation is rare in patients with asplenia (right isomerism). Associated anomalies of the abdominal IVC such as left-sided or duplicated IVC are frequent [55].
Get Clinical Tree app for offline access

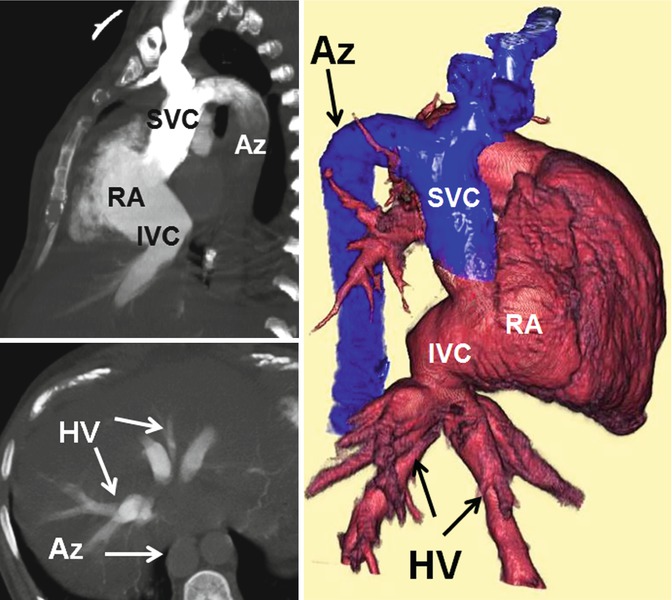
Fig. 26.15
Azygos (Az) continuation of inferior vena cava (IVC




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree