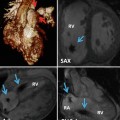
Fig. 7.1
The developing outflow tract in embryonic chicken hearts at stages 12, 24, 30, and 36 H/H. The area of the outflow tract (OFT) extends between distal ventricular groove (DVG) of the right ventricle and the junction with aortic sac at pericardial reflections and divided into the conus (proximal OFT shown in red) and the truncus (distal OFT in light blue). The OFT is divided into two parts, conus and truncus, and the junction between the two will be distal myocardial border (DMB). The images show that the OFT is initially mainly myocardial (red part) in its entirety, increasing in length up to HH24. The OFT myocardium, subsequently, shortens as a result of ventricularization, contributing to the trabeculated free wall, as well as the infundibulum, of the right ventricle (RV). Note the absolute reduction in the length of the OFT between 30 and 36 H/H stages, as well as the relative reduction in relation to the ventricles, which have increased in size by cardiomyocyte proliferation. The OFT has also been divided by septation into pulmonic and systemic outflows, and the aortic root has rotated to a posterior position, where it connects with the left ventricle (LV). The dotted line around the heart indicates the pericardium. A primitive atrium, LA left atrium, RA right atrium, SV sinus venosus, V primitive ventricle
OFT undergoes rotation during its remodeling. Rotation of the myocardium at the base of the OFT is probably essential to achieve normal positioning of the great arteries with respect to each other at the ventriculoarterial junction [9, 10]. In addition to abnormal OFT septation caused by neural crest cell defects, a spectrum of conotruncal anomalies with abnormally positioned great arteries may arise from a perturbation of myocardial rotation including tetralogy of Fallot (TOF), persistent truncus arteriosus, double outlet right ventricle (DORV), and transposition of the great arteries (TGA) [10, 11]. A short outflow tract as obtained experimentally through secondary heart field ablation may not allow a normal conotruncal rotation [4]. Although in conotruncal anomalies including TOF, the infundibular septum is not always short. It is believed that any time the RV shows a short outflow tract, a total or partial lack of conotruncal rotation and remodeling is inevitably present [9] (Fig. 7.2).
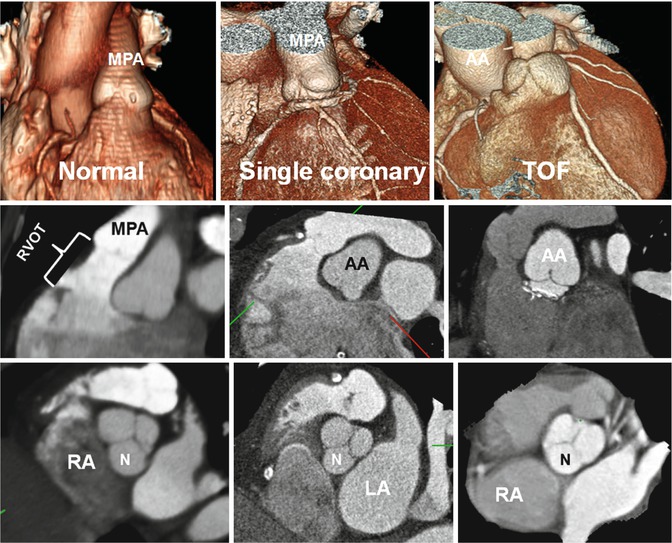

Fig. 7.2
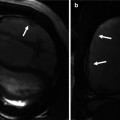
Variations in right ventricle outflow tract (RVOT) length. In normal example the pulmonary valve extends well above the coronary sinuses of the ascending aorta (AA). The RVOT has a vertical course, and the middle of noncoronary (N) sinus overlays the interatrial septum. In patients with single coronary artery arising from the right coronary sinus and tetralogy of Fallot (TOF), the RVOT is short, and the aortic root is rotated clockwise facing the right atrium (RA). The RVOT is thickened and narrowed in TOF. Although in conotruncal anomalies including TOF the infundibular septum is not always short, it is believed that any time the RV shows a short outflow tract, a total or partial lack of conotruncal rotation is present. LA left atrium, MPA main pulmonary artery
Anatomical Evaluation of RVOT
Imaging Techniques
Cardiac CT and MR allow comprehensive morphological and functional assessment of the heart within a single examination. Higher spatial resolution and availability of isotropic multiplanar data acquisition of cardiac CT angiography make it the preferred technique over current routine MR techniques for detailed anatomical study of the RVOT. Using new CT scanners, entire heart acquisition can be obtained in a short breath hold combined with thin slices (0.5–0.75 mm). This greatly reduces motion artifacts, and the thin collimation improves the depiction of small structures. Anatomical analysis of the RV can be performed with a dedicated ECG-gated right heart study or as part of CT coronary angiography [12, 13]. In the latter, most of the time sufficient attenuation for visualization of the right heart can be obtained by split-bolus injection in which an initial bolus of contrast medium (50–75 mL) is followed by 50 mL of a 70 %:30 % saline-to-contrast medium mixture and a 30-mL saline chaser at a rate of 4–5 mL/s [13]. A dedicated right heart examination with CT requires ECG gating/triggering and homogeneous enhancement of the right atrium and ventricle to an optimum Hounsfield unit of 350–400. Scan can be started early (i.e., main pulmonary artery triggering) to include the right heart only. For certain cases with congenital heart disease, a modified injection protocol using dual extremity contrast injections into the antecubital and femoral veins is described which provides homogeneous images of the right atrium and ventricle [14] (Chap. 10 for detail). Different sequences can be used to evaluate the RVOT morphology with MRI including MR angiography, dark/bright blood sequences, and cine images. In addition to routine short- and long-axis cine [ECG-gated cine balanced steady-state free precession (SSFP)] views, transaxial and sagittal images may help to show the abnormality. ECG-triggered, double inversion recovery fast (segmented or single shot) spin-echo sequence with blood suppression is a great technique for anatomical imaging of the outflow tracts and major vessels especially in patients with metallic implant artifacts or those with compromised renal function and contraindication to gadolinium-enhanced MRI.
Anatomical Landmarks
The RV in the normal heart is the most anteriorly situated cardiac chamber and marks the inferior border of the cardiac silhouette. In contrast to the near conical shape of the LV, the RV appears triangular when viewed from the side and crescent shaped when viewed in cross section [15–18]. The curvature of the ventricular septum places the RVOT antero-cephalad to that of the LV’s resulting in a characteristic “crossover” relationship between right and left ventricular outflows. This important spatial relationship can be lost in congenital heart malformations such as TGA. The overlap between left ventricular inlets and outlets puts the aortic outflow tract immediately behind the septum that separates it from the RV inlet, giving the “wedged” position of the aortic root.
Traditionally, the RV is divided into the sinus and conus parts, but in more recent decades, both the right and left ventricles have been described as having three components: the inlet, apical trabecular, and outlet portions (Fig. 7.3) [15–17]. In the analysis of congenitally malformed hearts, this tripartite concept is more useful than the traditional bipartite division. The inlet portion of the RV surrounds the leaflets of the tricuspid valve and supports its papillary muscles and tension apparatus. A distinguishing feature of the tricuspid valve is the direct attachment of its septal leaflet to the ventricular septum. The apical portion of the RV is characterized typically by heavy trabeculations. Distinguishing features of the outlet portion of the RV include:
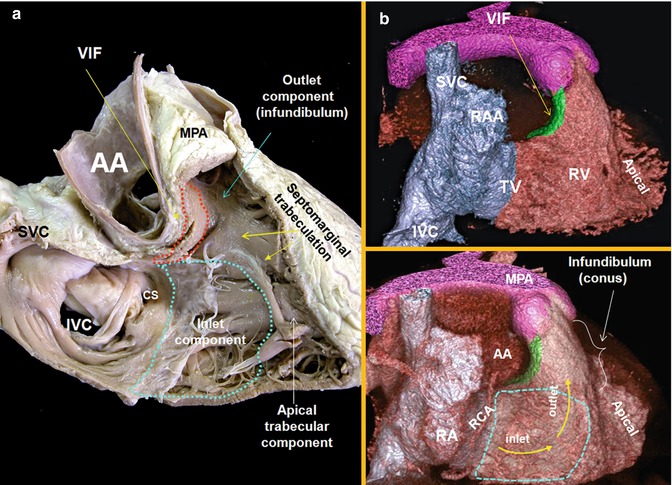

Fig. 7.3
Right anterior oblique views (two chambers) of the right heart are shown. (a) Cadaveric specimen. (b) Volume-rendered images of a right heart CT. The right ventricle comprises of three components: the inlet, apical trabecular, and outlet portions. The outflow tract, the infundibulum or conus, separates the tricuspid and pulmonary valves. The axis of the orifices of the inlet and outlet roughly forms an angle of 60°. The supraventricular crest is composite of the ventriculoinfundibular fold (VIF) cradled between the limbs of septomarginal trabeculation. It is separated from the right aortic sinus by the epicardial fat. AA ascending aorta, MPA main pulmonary artery, RA right atrium, RAA right atrial appendage, SVC superior vena cava, IVC inferior vena cava, CS coronary sinus
Pulmonary Infundibulum
The pulmonary infundibulum (conus) is a tubular muscular structure that supports the leaflets of the pulmonary valve. Its length, size, and angle vary. The size of the infundibulum is independent of the general size of the RV.
Supraventricular Crest
The posterior (paraseptal) wall of the infundibulum is formed by a prominent muscular ridge, known as the supraventricular crest (crista supraventricularis or ventriculoinfundibular fold) which separates the inlet and outlet components of the RV (Figs. 7.3, 7.4, and 7.5). This is in contrast to the LV where the aortic and mitral valves are in fibrous continuity. Although it looks like a ridge from the perspective of the RV cavity, the supraventricular crest is in fact an infolding of the ventricular wall (the ventriculoinfundibular fold) inserting into the ventricular septum. It is separated from the aorta by the epicardial fat, and any incision through it will lead outside the heart into the vicinity of the right coronary artery (Figs. 7.3 and 7.4). Only the central portion of its inferior most part between the limbs of the septomarginal trabeculation contributes to the interventricular septum (muscular outlet septum or conal septum) [17] (Figs. 7.4 and 7.5). In the normal heart, however, this area is exceedingly small and can hardly be distinguished from the septomarginal trabeculation by CT.
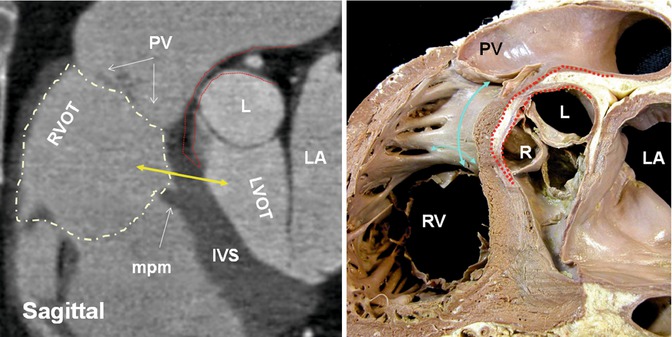
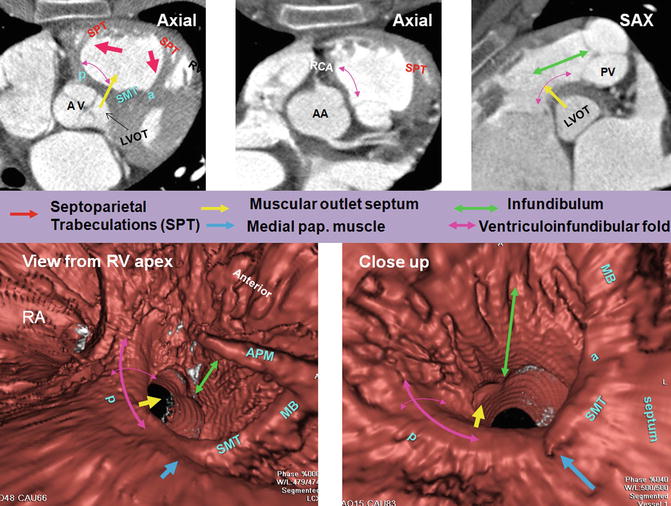

Fig. 7.4
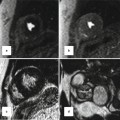
Sagittal views of CT and cadaveric specimen at the right ventricle outflow tract (RVOT). Note fat plane (dotted red line) extending between the posterior wall of the RV infundibulum (supraventricular crest) (blue arrows) and the anterior wall of ascending aorta making surgical resection of the infundibulum possible. The height of supraventricular crest varies from patient to patient; however, most of it is separated from the aorta by epicardial fat and can be surgically removed without entering the left ventricular cavity. Only the central portion of its inferiormost part may form part of the interventricular septum. The term supraventricular crest is replaced by ventriculoinfundibular fold, representing any muscular structure interposed between the attachments of the leaflets of the atrioventricular and arterial valves. The inferior central part of it is called outlet septum which is part of septomarginal trabeculation. The outlet septum above the level of medial papillary (double headed yellow arrow) muscle (mpm) is shown by yellow arrow. However, its true existence is questionable given the limited spatial resolution of CT compared with histological slices. PV pulmonary valve, L left aortic sinus, R right aortic sinus, RV right ventricle, LA left atrium, IVS interventricular septum, LVOT left ventricle outflow tract

Fig. 7.5
Right ventricle (RV) infundibulum. The posterior wall of RV infundibulum is the continuation of the infundibulum into the ventriculoinfundibular fold (supraventricular crest). It is a free-standing wall except its mid-inferiormost part which contributes to the interventricular septum also known as the outlet septum (yellow arrows). One slice above it (second axial cut) at the orifice of the right coronary artery (RCA) demonstrates extension of fat plane between the ascending aorta and RVOT. The RCA can be injured at the time of pulmonary valve surgery. The ventriculoinfundibular fold is located between the antero-cephalad (septal band) (a) and postero-caudal (parietal band) (p) limbs of the septomarginal trabeculations (SMT), and its width is shown by double-headed purple arrows. The cradle between the SMT limbs and the ventriculoinfundibular fold forms the supraventricular crest. Note smooth surface of ventriculoinfundibular fold compared to the rest of RV infundibulum which is trabeculated. The anterior limb runs superiorly into the infundibulum and supports the leaflets of the pulmonary valve (PV). Multiple muscular bundles that extend from the cephalad margin of the septomarginal trabeculation and run onto the parietal wall of the outflow tract are designated as the septoparietal trabeculations (SPT) (red arrows). AA ascending aorta, AV aortic valve, PUV pulmonary valve, MB moderator band, RA right atrium
Septomarginal Trabeculation
The septomarginal trabeculation is a prominent Y-shaped muscular strap reinforcing the septal surface. It bifurcates into anterosuperior and inferoposterior limbs (Fig. 7.6) which clasp the supraventricular crest. The anterosuperior limb extends along the infundibulum to the leaflets of the pulmonary valve. The posterior limb runs onto and overlays the ventricular septum, toward the right ventricular inlet, giving rise to the medial papillary muscle complex. The body of septomarginal trabeculation extends to the apex of the ventricle, where it gives rise to the moderator band and anterior papillary muscle before breaking up into the general apical trabeculations. The body of the septomarginal trabeculation is interventricular rather than supraventricular and when enlarged can appear as a bump on the septum on cross-sectional imaging. When hypertrophied, the septomarginal band can divide the RV into two chambers (double-chambered RV) [19].
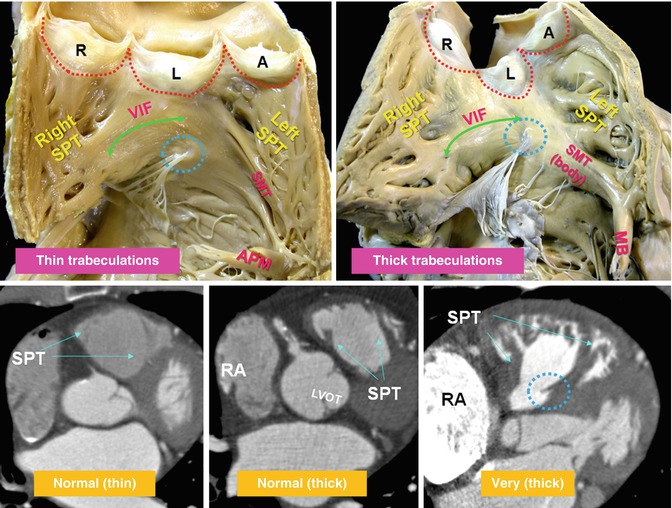

Fig. 7.6
Variation of trabeculations in RVOT. Upper panel: RVOT open-book dissection views. The septomarginal trabeculation (SMT) is a muscle strap plastered onto the septal part. The ventriculoinfundibular fold (VIF) extends between SMT and the pulmonary valve and forms the paraseptal wall of the RVOT. The septoparietal trabeculations (SPT) originate from the anterior margin of the SMT and run round the parietal quadrant of the endocardial infundibulum along the right and left septoparietal walls of the RVOT. These trabeculations vary in number (5–22 trabeculations) and thickness (2–10 mm). The SPTs can be flat or prominent and may be hypertrophied as in pulmonary hypertension or tetralogy of Fallot, contributing to muscular subpulmonary stenosis. The SMT continues to apex and turns into the moderator band (MB) and anterior papillary muscle. Blue circles demarcate medial papillary muscle. Lower panel demonstrates variable thickness of the SMT and its SPTs. Note marked thickening of the RVOT in the last image in a patient with pulmonary valve stenosis. The right atrium (RA) is markedly enlarged. A anterior, R right posterior, and L left posterior are pulmonary sinuses, LVOT left ventricle outflow tract, RA right atrium, red dotted line denotes margin of the pulmonary valve leaflets
Moderator Band
It is considered as part of the septomarginal trabeculation, supporting the anterior papillary muscle of the tricuspid valve and, from this point, crossing to the free wall of the ventricle. The moderator band incorporates the right atrioventricular bundle, as conduction tissue fibers move toward the apex of the ventricle before entering the anterior papillary muscle. It is usually located equidistant from the tricuspid valve and the apex and can be identified in 90 % of hearts. In 40 % the band is a short and thick trabeculation. The average thickness of the band is 4.5 mm, and its length is 16 mm, ranging from 11 to 24 mm [20]. The moderator band is supplied by branches of the left anterior descending (LAD) artery named the artery of the moderator band. The artery supplying the band makes anastomotic connections at the base of the anterior papillary muscle with branches of the right coronary artery.
Medial Papillary Muscle of the Conus
The medial (septal) papillary muscle of the conus presents in 82 % of the hearts, while in the rest, it is replaced by tendinous chords [21] (Figs. 7.4 and 7.6). It is a single papilla in 50 % and double in 30 %. It connects with the septal and anterior leaflets of the tricuspid valve. It represents an important surgical landmark for the location of the right bundle branch to avoid injury to the bundle during surgical correction of certain types of ventricular septal defects.
Pulmonary Valve
The pulmonary root is the part of RVOT that supports the leaflets of the pulmonary valve. It consists of three sinuses of Valsalva confined proximally by the semilunar attachments of the valvular leaflets and distally by the sinotubular junction. Different nomenclatures have been used to define the anatomical location of the pulmonary valve sinuses base on their spatial location in relation to the body of the heart itself (Fig. 7.7). Because of the semilunar shape of the pulmonary leaflets (similar to the aortic valve), this valve does not have a ringlike annulus. The sinotubular junction of the pulmonary trunk marks the level of the commissures between the annuli (Fig. 7.7). Compared to the aortic root, the pulmonary sinotubular junction is less obvious on CT images. A second junction exists at the ventriculoarterial junction. The bases of the sinuses within the ventricle cross the anatomical ventriculoarterial junction. The anatomical ventriculoarterial junction forms the annulus. The semilunar attachment of the valvular leaflets which forms the hemodynamic ventriculoarterial junction crosses the anatomical ventriculoarterial junction. The leaflets are thickened along their semilunar line of attachment. The fibrous interleaflet trigones are the areas of arterial wall proximal to the semilunar attachments of the leaflets and therefore are incorporated within the ventricular cavity. Their tips point toward the commissures. The musculature of the subpulmonary infundibulum raises the pulmonary valve above the ventricular septum to position the pulmonary valve as the most superiorly situated of the cardiac valves. This anatomical feature makes possible the safe resection of the pulmonary valve, including its basal attachments within the infundibulum from the rest of the RVOT.
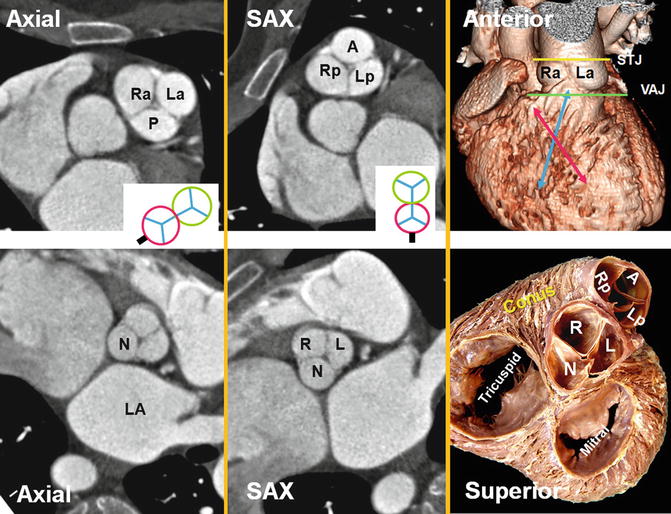

Fig. 7.7
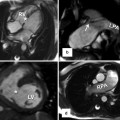
Pulmonary valve sinuses. When heart is viewed in attitudinal anatomical position as sitting in the thorax (i.e., axial views), the pulmonary leaflets and sinuses are seen to be posterior (P), right anterolateral (Ra), and left anterolateral (La). However, in relation to the heart (i.e., short-axis views), the pulmonary sinuses can be named anterior (A), left posterior (Lp), and right posterior (Rp). According to their relation to the aorta, sinuses will be left-facing, right-facing, and nonfacing. Same rule can be applied to the aortic valve as seen in the above examples. The relationship of the pulmonary and aortic valve (green and red circles) as well as their orientation in relation to the body (axial) and the heart (short axis, SAX) are drawn; the black line shows the location of the interatrial septum. Crossover arrangement between left and right ventricular outflow tracts (red and blue arrows respectively) is also shown. The term “pulmonary annulus” denotes the semilunar fibrous attachment of each of the pulmonary leaflets. They are much less sturdy than the aortic annuli. LA left atrium, N noncoronary sinus, STJ sinotubular junction (yellow line), VAJ ventriculoarterial junction (green line)
Arterial Supply and Anatomical Variants
The conotruncal structures are normally vascularized by anterior and posterior arterial branches from the right and left coronary arteries [22]. On the right side, the branches arise from the conal branch of the right coronary artery or directly from the aorta. On the left side, they arise from the LAD, the left main, or directly from the aorta. The right anterior conal branch is the most constant and conspicuous branch participating in the preconal circulation, also known as Vieussens’ arterial ring [22] (Fig. 7.8). This collateral intercoronary connection extends between the conus artery and first right ventricular branch (left anterior conus branch) of the LAD artery. The Vieussens’ arterial ring will become dilated when there is proximal LAD artery occlusion or, less frequently, RCA occlusion. Generally, three major collateral pathways at conotruncal level provide circulation between right and left coronary system in all congenital or acquired forms of one-sided coronary occlusion and are used as the basis for different classifications [23]. These three collateral circulation pathways include preconal (precardiac), retroconal (interarterial), and retroaortic. In coronary ostial atresia, because intercoronary collaterals develop very early in life, they can be large and overall angiographic appearance of the anomaly can be difficult to differentiate from congenital single coronary artery malformation. In congenital single coronary artery, the blood flow is always centrifugal from larger caliber arteries proximally to smaller ones distally. In ostial atresia the blood flows from the intact right or left coronary artery to the abnormal side via one or more collateral arteries whose caliber is smaller than that of the target vessels (Fig. 7.9). The incidence of a major coronary artery crossing the RVOT in TOF is between 5 and 12 % [24]. Preoperative recognition of such arteries may be important in reconstructive surgery of the RVOT (Fig. 7.10). Infundibular and preventricular branches should not be mistaken for a major coronary artery arising crossing the RVOT.
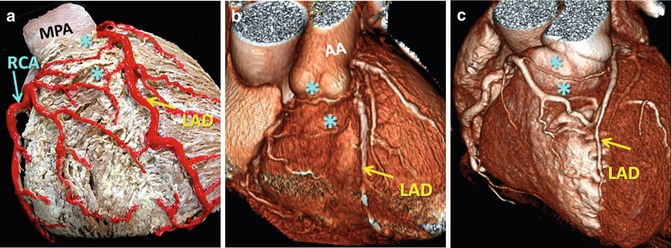
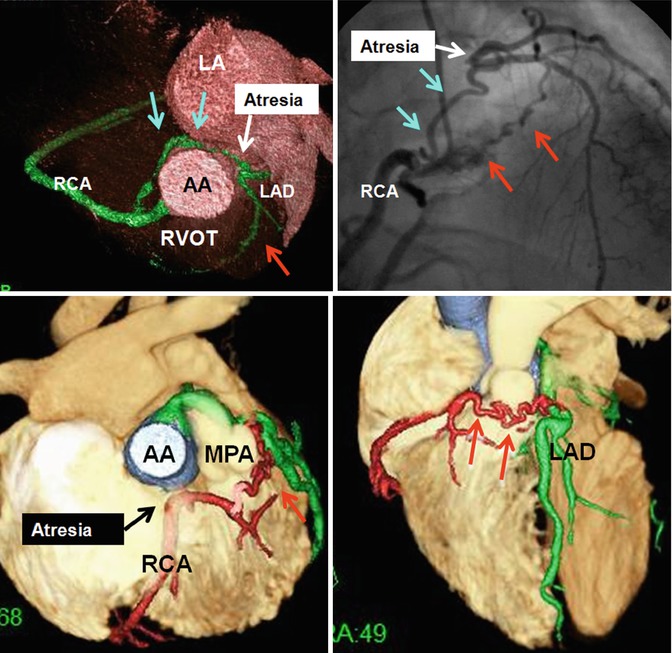
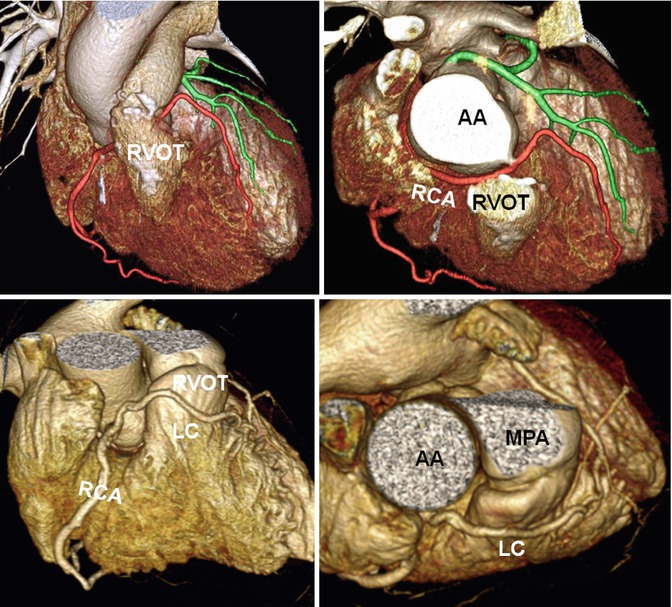

Fig. 7.8
Vieussens’ arterial ring; preconal arterial anastomotic rings between pulmonary conus branches arising from the RCA and the LAD artery are shown (a) participating in vascular supply to infundibulum as well as the anterior right ventricle. These collaterals may be enlarged in acquired obstructive (b) or congenital (c) coronary disease. AA ascending aorta, MPA main pulmonary artery, RCA right coronary artery, LAD left anterior descending artery

Fig. 7.9
Upper row shows left coronary ostial atresia with retroaortic (blue arrows) and preconal (red arrows) collaterals between the right and left coronary systems. Lower row shows right coronary ostial atresia with preconal (red arrows) collaterals between the right and left coronary systems in a patient with congenital pulmonary valve stenosis. AA ascending aorta, LA left atrium, LAD left anterior descending artery, MPA main pulmonary artery, RVOT right ventricle outflow tract, RCA right coronary artery

Fig. 7.10
Anomalous course of the coronary arteries next to the right ventricle outflow tract (RVOT). Upper row shows repaired RVOT in tetralogy. Anomalous course of the right coronary artery (RCA) behind the RVOT conduit is seen. Lower row shows a single RCA with preconal course of the left coronary (LC) artery. AA ascending aorta, MPA main pulmonary artery
Morphological Changes in Adult CHD
Major advances in cardiac surgery over the past 50 years have resulted in a marked increase in the number of patients with congenital heart disease reaching adulthood. In many cases initial surgery is indicated on the basis of echo, with catheterization for physiological assessment if required. CT and MR have a prominent role in follow-up, either to monitor changes during staged surgical repair or to look for complications which are common and many need imaging for diagnosis and surgical planning. However, it is not unusual to discover an RVOT malformation for the first time and without a history of past surgery.
RVOT Stenosis: Pre- and Postoperative Findings
RVOT stenosis is usually secondary to pulmonary valve diseases, but stenotic lesions at subvalvular or supravalvular levels are not uncommon. Causes of RVOT stenosis are listed in Fig. 7.11.
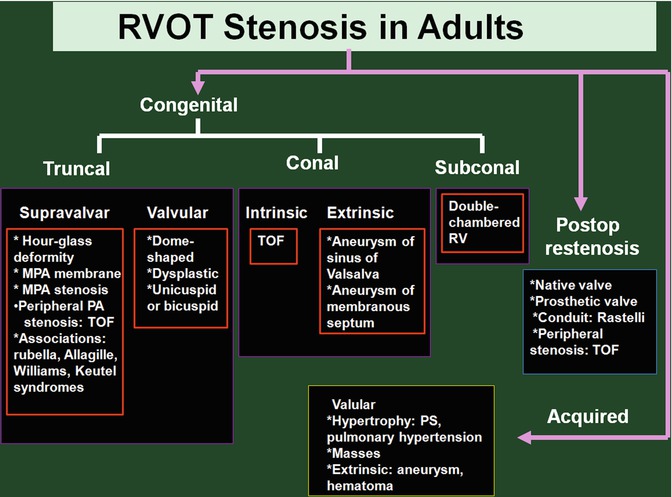

Fig. 7.11
Causes of RVOT stenosis in adults. TOF tetralogy of Fallot, MPA main pulmonary artery, PA pulmonary artery, PS pulmonary stenosis, RV right ventricle
Pulmonary Valve Stenosis
Isolated pulmonary stenosis (PS) is almost always congenital and many can be asymptomatic when first diagnosed. It is not unusual to suspect PS in a young patient on routine chest x-ray or CT by noticing enlarged main and left pulmonary arteries. With severe PS, symptoms of dyspnea, fatigue, chest pain, palpitations, and decreased exercise tolerance may occur. Three morphological types are described [18, 25–28] (Table 7.1). The most common type of congenital PS (40–60 %) is a dome-shaped pulmonary valve, which is characterized by a mobile valve and 2–4 raphes and incomplete separation of valve cusps due to commissural fusion resulting in funnel with a small circular orifice (Fig. 7.12a). The line of basal attachment of the domed valve is not semilunar; instead, the sinuses are shallow and the line attachment appears somewhat circular. A waist-like narrowing of the sinotubular junction may be seen is some cases. Dysplastic pulmonary valve is the second most common PS (20–30 %) and is associated with immobile thickened cusps and in some cases a hypoplastic ventriculoarterial junction [26] (Fig. 7.12b). Cauliflower-like myxomatous thickening is limited to the free margin of the leaflets, and the proximal part of leaflets is intact. The commissures are not fused, the sinuses are deep, and the lines of attachment are semilunar, all as seen in normal hearts. The semilunar attachment of the pulmonary valve leaflets is an essential feature for normal function of the valve. Shallow attachment and lack of “height” of the overall valvular apparatus can cause pulmonary stenosis due to limiting mobility of the free edge of the leaflets even in the absence of commissural fusion. Of those cases with PS who require active treatment whether interventional or surgical, dysplastic valves would be far more common. Milo et al. [25] described a third morphology of PS with deep “bottle-shaped” sinuses and an hourglass deformity due to supravalvular narrowing at the sinotubular junction (Fig. 7.12c). Although the later morphology is reported in 16 % of patients with congenital PS, it is not accepted as a separate variant by every investigator [26]. Dome-shaped valve with dysplastic leaflets is another uncommon variant. Different morphologies can be equally distinguished with cardiac MR and CT angiography. Bicuspid or multicuspid valve is rare [27] (Fig. 7.13). In bicuspid valve, one leaflet can be larger containing a shallow raphe or both leaflets may be equal in size. Stenosis and post-stenostic dilatation are common. Compared to bicuspid valve, quadricuspid pulmonary valve is usually asymptomatic. Mild pulmonary regurgitation (PR) is not uncommon. Congenital variations can be isolated but are often associated with other congenital heart anomalies. For example, tetralogy of Fallot can be associated with a bicuspid pulmonary valve. Congenital pulmonary valve anomalies can also be associated with extracardiac anomalies as in Noonan syndrome and LEOPARD syndrome, which is often associated with a dysplastic pulmonary valve.
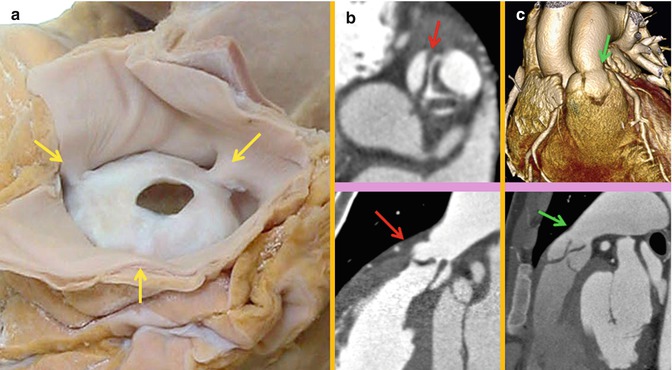
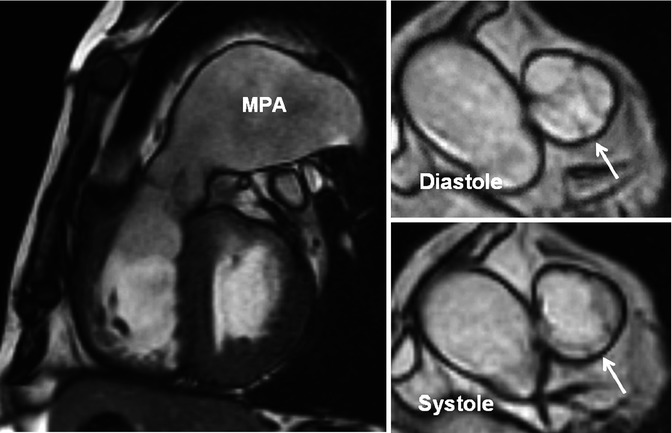
Table 7.1
Congenital pulmonary valve stenosis
A. Dome-shaped |
Very common: 80–90 % of all congenital right ventricle outflow tract lesions |
Low familial inheritance |
2–4 raphes but no separation into valve cusps |
Treatment: balloon valvotomy |
B. Dysplastic |
10–20 % |
Trileaflet with markedly thickened leaflets |
Associations: hypoplastic ventriculoarterial junction. Noonan’s syndrome |
Treatment: partial or total valvotomy, a transannular patch |
C. Bicuspid/quadricuspid |
Rare |
Usually asymptomatic |
Common with other congenital heart diseases |

Fig. 7.12
(a). Dome-shaped pulmonary valve viewed from the arterial aspect. It is characterized by narrow opening and incomplete separation of the valve cusps. The fused commissures (yellow arrows) pull the sinotubular junction toward the central circular orifice. (b) Axial and sagittal CT appearance of a dysplastic pulmonary stenosis. Club-shaped myxomatous thickening (red arrows) is limited to the free margin of the leaflets, and the proximal part of leaflets appears intact. Note the trileaflet thickened valve and no commissural fusion with hypoplastic ventriculoarterial junction. In dysplastic pulmonary valve, there are three distinct cusps and no commissural fusion. (c) Volume-rendered and sagittal CT images in a patient with LEOPARD syndrome and pulmonary stenosis (arrows). Note mild thickening of the valve leaflets and narrowing at sinotubular junction giving an hourglass appearance

Fig. 7.13
A 51-year-old male with mild pulmonary regurgitation and pulmonary hypertension. Long-axis and short-axis MR images of a quadricuspid valve are shown. The valve is shown at two different phases of cardiac cycle. One rudimentary extra cusp between the left posterior and anterior (nonfacing) cusps (arrows) is seen. The right ventricle is hypertrophied and the main pulmonary artery (MPA) is dilated
Chronic PS results in RV hypertrophy, especially at the RVOT. When prominent, RVOT hypertrophy can lead to secondary dynamic subvalvular stenosis. Distinguishing between valvular stenosis and subvalvular dynamic stenosis secondary to infundibular hypertrophy can become challenging. Subvalvular dynamic obstruction (late systolic stenosis), in fact, often accompanies severe valvular PS and is characterized by a late-peaking jet in MRI similar to that of dynamic LV outflow obstruction. PS can also result in post-stenotic dilatation of the pulmonary trunk and left pulmonary artery. For symptomatic patients with dome-shaped pulmonary valve, balloon valvuloplasty is indicated when a peak instantaneous gradient >50 mmHg is present [28]. A successful procedure is defined by final peak gradient of <30 mmHg and is obtained in >90 % [29]. If the valve is dysplastic, surgery is more likely to be required; if there is annular or pulmonary trunk hypoplasia, a transannular patch may become necessary. In patients with PS and significant pulmonary regurgitation, valve replacement is required. Mechanical valve replacement is used rarely because of thrombosis issues. Bioprosthetic valves and pulmonary homografts are preferred [30].
Tetralogy of Fallot
TOF consists of a large nonrestrictive subaortic ventricular septal defect (VSD), dextroposed aorta riding up over the septal defect, and RVOT obstruction (Fig. 7.14). TOF without PS is called Eisenmenger complex (Fig. 7.15). Subpulmonary stenosis, which is an essential part of TOF, is mainly due to anterosuperior malalignment of the muscular outlet septum relative to the limbs of the septomarginal trabeculation, coupled with thickened septoparietal trabeculations [17, 31, 32] (Fig. 7.14). Stenosis can also occur at subpulmonic level by hypertrophy of the septomarginal trabeculation or the moderator band. This gives the arrangement often described as “two-chambered right ventricle.” The subpulmonary infundibulum itself varies markedly in length and can sometimes be short especially in Eisenmenger complex (Fig. 7.2). In most other instances of TOF, the narrowed infundibular chamber is normal in length but sometimes has considerable length. Absent pulmonary valve syndrome occurs in less than 3–6 % of TOF patients [17]. This syndrome is associated with significant pulmonary artery dilatation and airway compression. Pulmonary atresia in TOF is also due to severe deviation of the outlet septum. However, isolated pulmonary atresia can rarely occur as a result of valve imperforation rather than severe stenosis. In pulmonary atresia blood supply to the right and left pulmonary arteries (if not atretic) will be provided by a large patent ductus arteriosus or multiple aortopulmonary collateral arteries. Extensive reconstructive surgery is required in extreme cases.
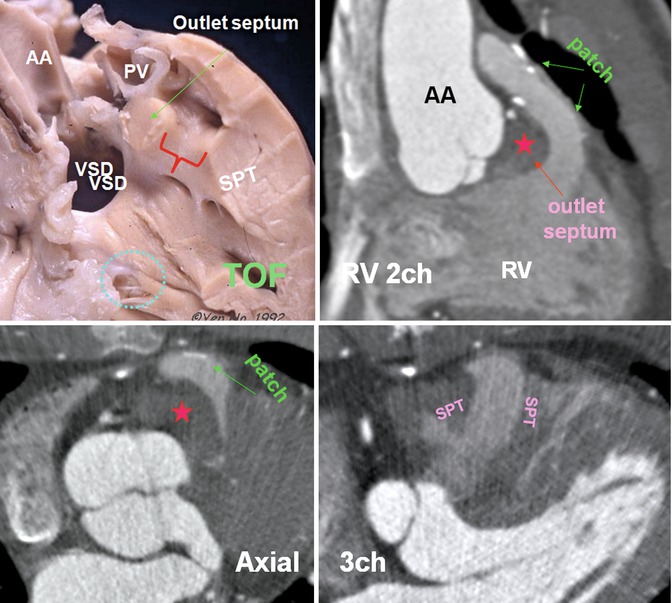
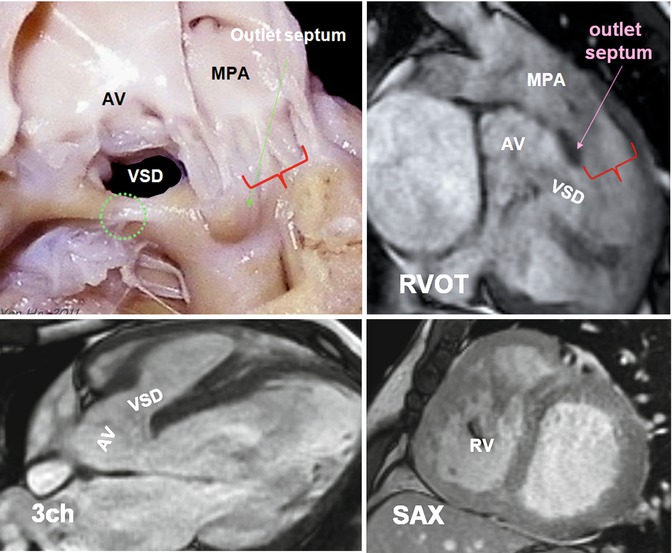

Fig. 7.14
Cadaveric specimen demonstrates phenotypic features of tetralogy of Fallot (TOF) including a large nonrestrictive subaortic perimembranous ventricular septal defect (VSD) with the aorta overriding the septal defect. The muscular outlet septum is displaced antero-cephalad to the limbs of the septomarginal trabeculation, and there is hypertrophy of the septoparietal trabeculations (SPT). Subpulmonary obstruction (red bracket) is generally produced between the muscular outlet septum and the hypertrophied SPTs. There is continuity between the leaflets of the aortic and tricuspid valves in the postero-inferior margin of the VSD. Note the VSD is located anterior to the medial papillary muscle (blue circle). CT images show repaired TOF with markedly thickened outlet septum (red stars) and the SPT. Status post transpulmonary patch surgery covering the anterior wall of the RVOT. In this patient the RVOT is long. The ascending aorta (AA) is dilated. The muscular outlet septum is located cephalad to the SPTs. PV pulmonary valve, RV 2ch right ventricle two chamber, 3ch three chamber

Fig. 7.15
Cadaveric specimen demonstrates phenotypic features of Eisenmenger complex. A subaortic ventricular septal defect (VSD) is shown. The outlet septum is mildly displaced superiorly but without causing subpulmonary obstruction (red bracket). Note the VSD is located anterior to the medial papillary muscle (green circle). MR images are obtained in a 35-year-old male with unrepaired tetralogy. Subaortic VSD and aortic overriding are shown. Outlet septum is superiorly displaced. There is no subpulmonary stenosis (red bracket). The right ventricle (RV) is thickened wall and appears mildly dilated. AV aortic valve, MPA main pulmonary artery, RVOT right ventricle outflow tract, SAX short axis, 3ch three chamber
Patients with TOF have remarkable intrinsic histological abnormalities and reduced elasticity in both ascending aorta and pulmonary artery, and it appears that TOF repair does not improve these abnormalities [33, 34]. Aortic root dilation with or without aortic regurgitation is common [33]. Cardiac MRI or CT can address these major clinical implications (Fig. 7.14). The concept of aortic overriding is shown in Fig. 7.16. Note that mild overriding above the ventricular septum can be seen in normal instances. Greater than 50 % overriding falls into definition of DORV subgroup. However, in TOF there is always fibrous continuity between the anterior mitral leaflet, while in DORV this may not be the case (Fig. 7.16). The concept of dextroposition is shown in Fig. 7.17.
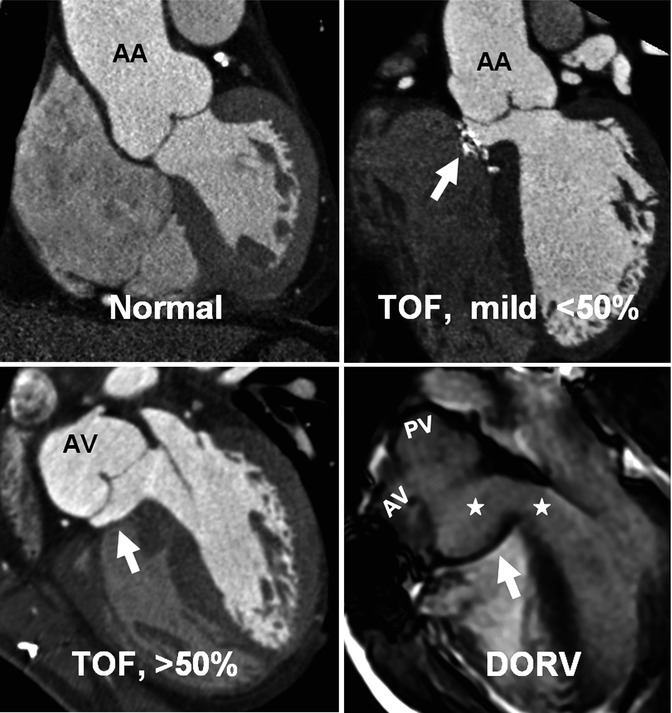
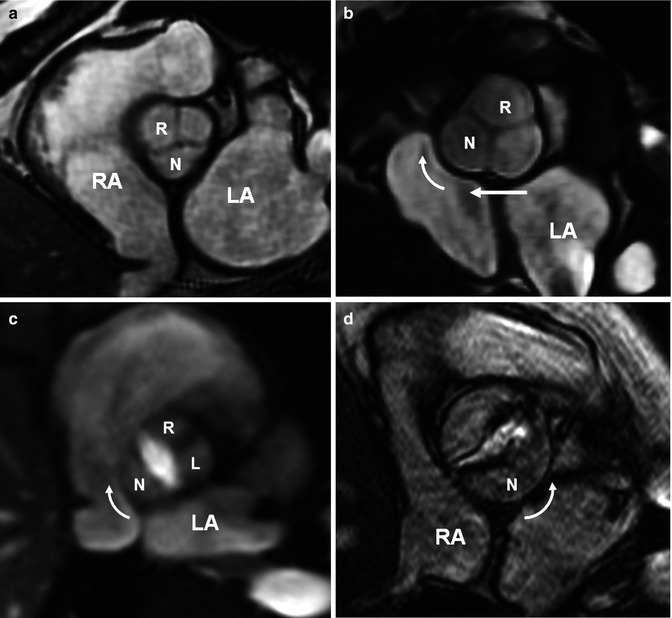

Fig. 7.16
The concept of aortic overriding. Coronal views of the heart at the level of the aortic root demonstrate the relationship of the aorta to interventricular septum. Mild dextroposition of the aorta is normal. When it overrides the septum greater than 50 %, it falls in definition of DORV. However, in this example aortic–mitral fibrous continuity which is essential for diagnosis of TOF still exists. In DORV both aortic valve (AV) and pulmonary valve (PV) arise from the right ventricle, and the pulmonary valve is usually located on the left side of the aorta. This will result in lack of fibrous continuity between the mitral and aortic valves as shown in this image. Arrows point to patch repaired VSDs. Stars denote left ventricle baffle. AA ascending aorta

Fig. 7.17
The concept of dextroposition. The term dextroposition indicates that there is specific anatomical evidence that the aortic root is rotated in a clockwise direction (looking from below) and is partially transposed to the right. (a) Normal heart with normal aortic root showing the noncoronary aortic sinus (N) facing the interatrial septum. In normal patients mild clockwise rotation of the aortic root toward the right atrium (RA) is not unusual. (b) Typical changes of the aortic root in tetralogy of Fallot (TOF) including clockwise rotation (curved arrow) and rightward translation (straight arrow). The root is also dilated causing aortic regurgitation in many adult TOF cases. Aortic root rotation is not limited to TOF and is seen in many conotruncal anomalies. (c, d) Demonstrate changes of the aortic root in two patients with bicuspid aortic valve, one with a raphe between the right (R) and the left (L) sinuses (c) and second without the raphe (d). Clockwise rotation is seen in c and counterclockwise rotation in (d). (d) Shows aortic stenosis. LA left atrium
Double-Chambered Right Ventricle
Double-chambered RV (DCRV) is characterized by subinfundibular stenosis due to aberrant hypertrophied septomarginal trabeculations or abnormal moderator band that divides the RV cavity into a proximal high-pressure and a distal low-pressure chamber [19, 35, 36] (Fig. 7.18). The severity of the DCRV stenosis tends to increase with time [36]. DCRV is usually associated with a perimembranous VSD. MRI and CT are usually diagnostic, identifying the degree and location of the obstruction and the presence of a VSD. The degree of stenosis can be best quantified with MR phase-contrast techniques. The indications for surgery in DCRV are similar to those for pulmonary valve stenosis (peak gradients >50 mmHg). Muscular resection and correction of VSD have excellent long-term results and low rates of recurrence [37].
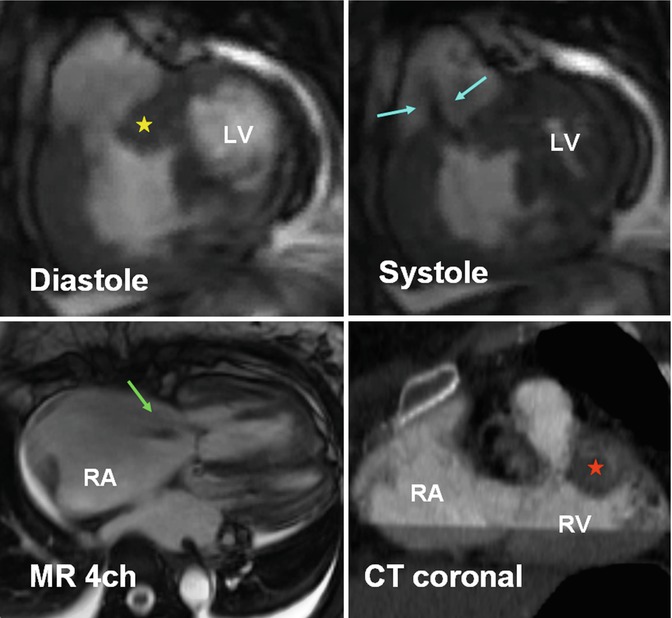

Fig. 7.18
A 48-year-old female with double-chambered right ventricle (RV). Severe RVOT stenosis is seen as a result of thickened septomarginal (yellow star) and septoparietal (red star) trabeculations. Jet flow is seen in systole (blue arrows). Thickened wall RV inlet is shown. The right atrium (RA) and the RV are enlarged, and there is mild to moderate tricuspid regurgitation (green arrow). LV left ventricle
Post-RVOT Repair Changes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree