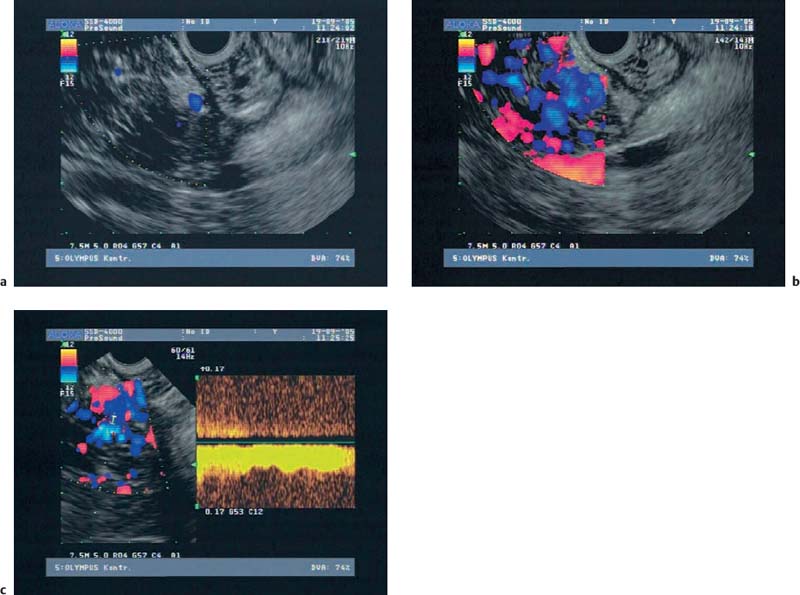
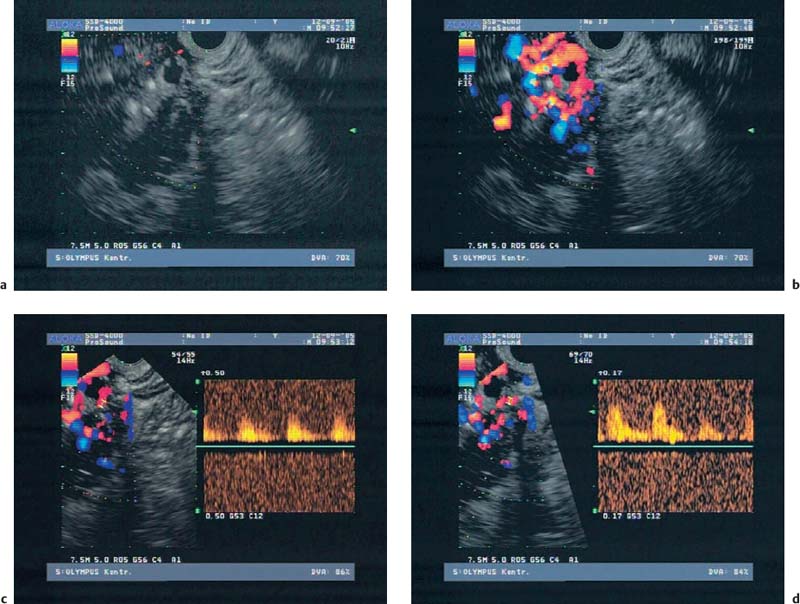
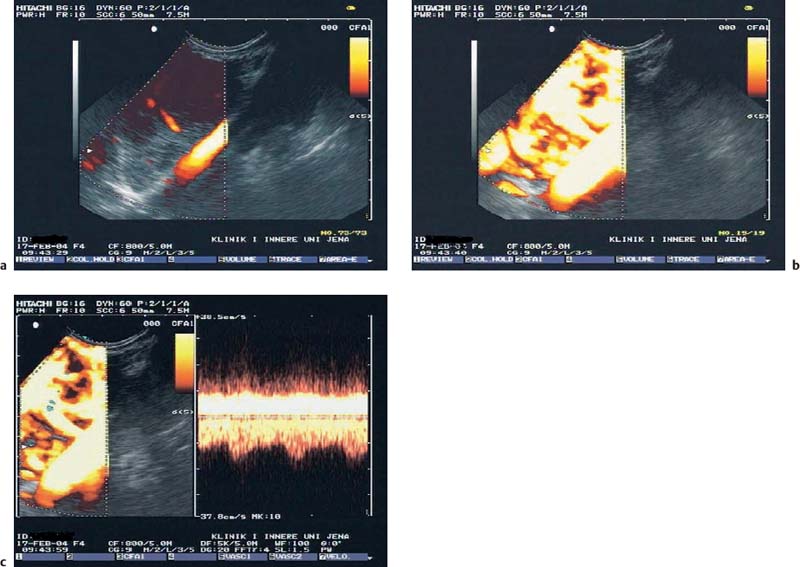
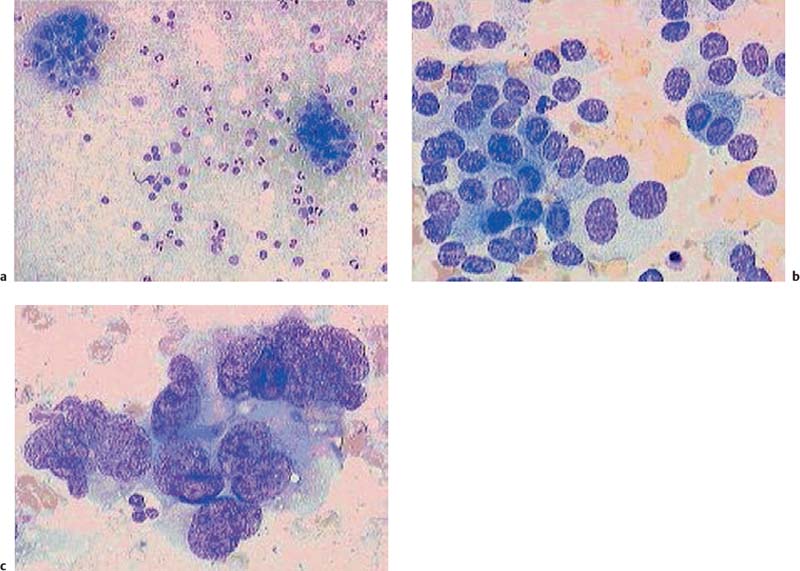
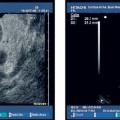
22 Contrast-Enhanced EUS The potential of contrast-enhanced endoscopic ultrasound (EUS) to detect pancreatic lesions that are not identified with conventional endoscopic ultrasound is limited. This situation is also unlikely to change, since it is difficult to improve the local resolution of endoscopic ultrasound, and with correct positioning of the scope and mastery of the examination technique, even structures only a few millimeters in size can be clearly recognized. Characterization of Malignancy The primary aim in using contrast enhancement in endoscopic ultrasound, therefore, is to improve the assessment of malignancy in pathological changes by optimizing the imaging ofvessel distribution. Endoscopic ultrasound with amplified echo signals has been found helpful in differentiating between benign and malignant pancreatic tumors, although a definitive conclusion cannot be reached in every case. Initial attempts to diagnose malignant areas in cases of chronic pancreatitis have been very promising. Above and beyond this, however, it has become apparent that with improved equipment and classification of the pancreatic vascular structures, the quality of malignancy assessment in pancreatic tumors can be greatly improved. The examination of patients with undiagnosed pancreatic masses has shown that pancreatic adenocarcinomas have a few arteries with an irregular configuration and that focal pancreatitis demonstrates both veins and arteries with a regular treelike distribution (Figs. 22.1 and 22.2). Endoscopic ultrasound is also useful for clarifying less common localized pancreatic growths. It is particularly valuable in cases of hypervascularization of microcystic pancreatic adenomas or neuroendocrine tumors, as well as macrocystic ones, although these are less well delineated (Figs. 22.3 and 22.4). The lesions can be diagnosed relatively reliably using color duplex endoscopic ultrasound and contrast-enhanced sonography. It is essential to make a differentiated diagnosis when there are mixed pancreatic tumors with different degrees of malignancy and in cases of hypervascularized pancreatic metastases, which are relatively often diagnosed using modern imaging, but which play a lesser role in determining the prognosis. In cases of hypervascularized pancreatic metastases, it is also important to take into consideration the possibility that they are metastases from renal adenocarcinoma, melanoma, small cell bronchial carcinoma, or mammary carcinoma.1 Fig. 22.1a–c Chronic pancreatitis a No evidence of vascularization on color Doppler imaging before contrast administration. b Evidence of long, regular blood vessels, homogeneously distributed, after contrast administration. c Evidence of arterial and venous signals on Doppler imaging. Fig. 22.2a–d Pancreatic carcinoma a No evidence of vessels on color Doppler imaging before contrast administration. b Evidence of short, irregular vessels with an inhomogeneous distribution after contrast administration. c, d Evidence of exclusively arterial signals on Doppler imaging. With the help of EUSfigguided fine-needle aspiration, endoscopic ultrasound represents an important tool in the diagnosis of pancreatic tumors. Preliminary data indicate that it is already possible today to do without histological specimens and rely on cytological specimens. This hypothesis is currently being tested—for example, at Topalidis’s cytology laboratory in Hanover, Germany (who kindly provided the images for Fig. 22.4) Fig. 22.3a–c Neuroendocrine tumor a Evidence of vessels on power Doppler imaging, even before contrast administration. b Evidence of a long, regular vessel structure after contrast administration. c Evidence of both arterial and venous signals on Doppler imaging. Fig. 22.4a–c Cytological images (courtesy of T. Topalidis, Hanover). a Chronic pancreatitis. b Undifferentiated pancreatic carcinoma. c Neuroendocrine carcinoma. Contrast-enhanced transabdominal ultrasound techniques were recently introduced. Administration of liver-specific microbubbles using Levovist makes it possible to detect metastases smaller than 1 cm and improves the ability to differentiate between benign and malignant lesions.3–5 Due to the complexity of the (intermittent) examination technique, with a high mechanical index and bubble destruction, the method was found to be difficult to use routinely. More recent advances with SonoVue combined with low mechanical index techniques have improved the transabdominal use of contrast-enhanced techniques, allowing real-time imaging with or without three-dimensional reconstruction.6 Several studies have been carried out to assess the potential role of contrast-enhanced endoscopic ultrasonography. Initial data on the practicalityofusing first-generation contrast agents were published by Bhutani et al.,7 who were able to demonstrate better imaging of vessels in swine after administration of Levovist. It should be noted that only one study has been conducted todateusing second-generation contrast agents, and no research has yet been published on contrast-specific software. Recognizing malignant neoplasia in chronic pancreatitis has always been difficult. The gold standard is still surgery. Although the introduction of EUS-guided fine-needle aspiration has made this easier, an improved noninvasive method of detecting malignant tissue would be preferable. In a recently published study, contrast-enhanced EUS was used to distinguish between benign and malignant pancreatic lesions. High sensitivityand specificity rates are possible when strong inclusion criteria are used. Isovascularity or hypervascularity as a sign of nonductal adenocarcinoma showed a sensitivity of 100%, a specificity of 90%, and an accuracy rate of 93.8%. Hypovascularity as a sign of ductal adenocarcinoma showed a sensitivity of 90%, a specificity of 100%, and an accuracy rate of 93.8%. Using this method, there is no real difference between endoscopic and transabdominal ultrasound. The only difference that needs to be taken into account is that the transabdominal approach is limited due to poor visibility of the lesion in ≈ 9% of patients. The main factor limiting differentiation using the global contrast-enhancing effect is that patients with chronic pancreatitis have to be excluded.8 This topic is further discussed in Chapters 8 and 18. When not only global enhancement effects of the contrast agent but also advanced vessel analysis are used for differentiation, it is possible to obtain reliable results even when patients with chronic pancreatitis are included. In patients with pancreatic cancer, contrast enhancement only shows arterial microvessels, whereas both venous and arterial microvessels are displayed after contrast enhancement in patients with chronic pancreatitis. We have used this effect in a study which produced the best differentiation results currently available. The study compared conventional EUS with contrast-enhanced EUS plus advanced microvessel analysis. Using conventional EUS criteria, the sensitivity for pancreatic cancer was 73% and the specificity was 83%. With contrast-enhanced EUS, the sensitivity increased to 91%, identifying 51 of56 patients with malignant pancreatic lesions. In 28 of 30 patients with chronic inflammatory pancreatic disease, the diagnosis was correctly recognized, giving a specificity value of 93.3%.9 These figures remained the same in a second study including 194 patients.10 As the method relies on the current resolution available with color Doppler, it is possible that improved equipment might in the future lead to uncertainties, with a few venous microvessels also becoming visible in patients with pancreatic cancer. To obtain a second criterion for distinguishing between focal pancreatitis and pancreatic carcinoma, we investigated the resistance index of the arterial microvessels shown on contrast enhancement. The study identified a cut-off point for the arterial resistance index of 0.7 in pancreaticcancer. A resistance index of more than 0.7 indicates pancreatic cancer, whereas a figure below 0.7 indicates chronic pancreatitis.11 As in studies using contrast-enhanced transabdominal ultrasound,12–15 ductal adenocarcinoma of the pancreas was found to be hypoenhancing in an EUS study by Hirooka et al.16
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine