Normal Anatomy
The sinuses of Valsalva are three adjacent dilatations or pouches of the aorta that have a cloverleaf configuration and lie above the level of the aortic valve but below the tubular portion of the ascending aorta. The sinotubular junction is where the sinuses of Valsalva meet the tubular segment of the ascending aorta. The right sinus of Valsalva is located anteriorly, and the left sinus of Valsalva is located more posteriorly and to the left. The right and left sinuses of Valsalva give rise to the right and left coronary arteries, respectively. There is also a noncoronary sinus called the posterior sinus of Valsalva , which in the normal configuration does not give rise to a vessel. The coronary arteries arise from the superior portions of the sinuses of Valsalva, just below the level of the sinotubular junction. The left main coronary artery (LMCA) normally arises more superiorly than the right coronary artery (RCA).
The proximal portion of the left coronary artery (LCA) is the LMCA. This segment is short (between 5 mm and 10 mm) and usually bifurcates into the left circumflex (LCx) and left anterior descending (LAD) arteries. The LMCA may trifurcate into LCx, LAD, and ramus intermedius in about 20% of people. The LAD usually descends in the anterior interventricular groove along the interventricular septum to the apex. The LAD gives rise to septal branches that course into the interventricular septum, supplying the superior two thirds of the septum. Diagonal branches arise from the LAD and descend toward the lateral margin of the left ventricular wall, supplying the anterior and sometimes anterolateral walls of the left ventricle. The septal branches may be difficult to see with cardiac computed tomography (CT), but the diagonal branches are consistently visualized. The diagonal branches are numbered sequentially (D1, D2, etc.).
The LCx branch descends in the left atrioventricular (AV) groove between the left atrium and left ventricle to supply obtuse marginal branches. There are a variable number of obtuse marginal branches, which supply the posterior and posterolateral walls of the left ventricle. The obtuse marginal branches are numbered sequentially (OM1, OM2, etc.).
The continuation of the LCx at this point can be variable. In left-dominant patients (10%) it travels all the way around the posterior wall of the heart, supplying both the posterior descending artery (PDA) and the posterior left ventricular branch, but more commonly it terminates as a small branch in the AV groove or continues as a small terminal posterior left ventricular branch.
The RCA originates from the right sinus of Valsalva at a slightly lower level than the LCA. The RCA then passes to the right, posterior to the pulmonary artery and under the right atrial appendage before descending in the anterior right AV groove between the right atrium and the right ventricle. In about 50% of patients the first branch from the RCA is the conus branch, which passes anteriorly and superiorly to supply the pulmonary outflow tract wall. The conus branch may also arise separately from the aorta or have a common origin from the aorta with the RCA. More rarely the conus branch may arise from the LCA. In 55% of patients the sinoatrial nodal artery is the next branch from the RCA. This vessel arises a few millimeters distal to the origin of the RCA and passes posteriorly toward the superior vena cava near the top of the interatrial septum. In 45% of patients the sinoatrial nodal artery arises from the proximal LCx. This is followed by two to three right ventricular wall branches. The acute marginal branch is the first large branch, which occasionally continues to the apex. In 70% of patients the RCA passes down the right AV groove to the crux of the heart, where it gives off the PDA and the posterior left ventricular branch. Approximately 20% of patients have a codominant supply, in which the PDA originates from the RCA but branches from the LCx also supply this territory. The PDA gives off inferior septal perforating branches which are not well visualized on cardiac CT.
Anomalies of the Coronary Arteries
Congenital abnormalities of the coronary arteries are uncommon, with an incidence in the general population of approximately 1% to 2%. They are often found incidentally at angiography or autopsy, and many are of no clinical significance. However, a minority are an important cause of symptoms, including chest pain, palpitations, dyspnea, and sudden death. In patients with congenital heart disease there is an increased incidence of anomalies reported to be between 3% and 36%.
In the past, catheter angiography was used to diagnose and evaluate coronary anomalies, but complex anomalous courses may be difficult to interpret and the angiographer may be unable to find the location of the ostium of the anomalous vessel. Cardiac CT has proven highly accurate in the depiction of such anomalies and has become the noninvasive imaging gold standard.
Symptoms of Coronary Anomalies
Coronary anomalies have been associated with sudden death in young (<35 years) athletes, particularly those in whom the coronary artery arises from the wrong aortic sinus of Valsalva. Exercise-induced episodic myocardial ischemia is believed to be the cause of death in these patients. Most coronary anomalies are surgically correctable once identified, and thus sudden death in athletes may be preventable in many cases. Death typically occurs during or after 30 minutes of strenuous exercise. Antemorbid cardiovascular symptoms may be present in approximately one third of these patients. Episodes of syncope, dizziness, palpitations, and exertional chest pain have been reported in athletes who subsequently died suddenly, with symptoms in retrospect occurring up to 2 years before death. Symptoms of syncope and chest pain are more strongly associated with anomalous LMCA than anomalous RCA. Overall, up to one third of patients who die from an anomalous coronary artery have a history of exertional syncope or chest pain. Electrocardiograms (ECGs) may be normal or demonstrate premature ventricular contractions. Results of exercise stress tests performed before death in these athletes are frequently normal without ST-segment or T-wave changes, arrhythmias, or cardiac symptoms.
Pearl
Normal results for a maximal exercise stress test do not alter the likelihood of a coronary anomaly being present.
Several theories have been proposed to explain the mechanism of myocardial ischemia and sudden death in these patients. Pathologically, in patients with sudden cardiac death caused by anomalous coronary arteries, the origin of the anomalous vessel may have a fibrous ridge with an acute angle, and the orifice of the vessel is typically slitlike. Flaplike closure of the abnormal orifice and compression of the vessel between the aorta and pulmonary trunk may contribute to reduced myocardial perfusion, particularly during exercise. This may commence a cascade of distal myocardial ischemia, ventricular tachycardia, ventricular fibrillation, and subsequent cardiac arrest. Endothelial damage with resultant coronary spasm or thrombosis, or both, at the site of anomalous vessels has also been suggested. Data from necropsies argue against the thrombosis theory because intraarterial thrombosis is rarely found in patients with sudden death and anomalous coronary arteries. By contrast, evidence of ischemic events is often identified at necropsy.
Pearl
The very proximal course of the anomalous vessel may be intramural within the myocardium. This is associated with a higher incidence of sudden death. Intramural courses allow for potential treatment with unroofing procedure of the anomalous vessel.
Pearl
Anomalous LCAs from the right sinus of Valsalva may have an intramyocardial course proximally (through the ventricular septum, resurfacing in the anterior interventricular groove). Such configurations are considered to be associated with a higher incidence of sudden death.
Congenital coronary anomalies are more commonly found as a cause of death in highly trained athletes than in nonathletes. The highest risk for death is in the first 30 years of life, and the risk increases if the aberrant coronary artery supplies a large area of myocardium. Because of the inherent lack of sensitivity of clinical examination, ECG, and exercise stress tests, these anomalies often become known only after death. In those patients who do have symptoms, the vast majority are exertional. In young trained athletes with exertional symptoms, the threshold for investigation of possible coronary anomalies should be low, given the catastrophic results that may occur if a malignant coronary anomaly is missed. Echocardiography has the ability to identify the location of the right and left coronary artery orifices in about 95% of young athletes. Failure to identify the proximal right and left coronary arteries at echocardiography in a young athlete with exertional symptoms should be followed by a second test to more clearly delineate the coronary artery origins, typically cardiac CT. Anomalous coronary arteries may also contribute to patient morbidity and mortality by increasing the incidence of complications during cardiac surgery and by increasing the technical difficulties encountered during angiography. There remains controversy over whether there is an association between coronary anomalies and increased risk for atherosclerotic disease.
Classification of Coronary Artery Anomalies
Coronary artery anomalies may be subdivided into three broad categories:
- 1.
Anomalous aortic origin with benign course
- 2.
Anomalous aortic origin with potentially “malignant” course
- 3.
Anomalous origin from the pulmonary artery (serious)
High Takeoff of Coronary Arteries From Ascending Aorta
The coronary artery ostia may have abnormal takeoffs from the ascending aorta, which subsequently course in a normal direction. The most common is a “high-takeoff” coronary artery, which implies that the right or left coronary artery arises above the sinotubular junction (arises from the ascending aorta). This may be present in up to 6% of the population. This usually occurs a few millimeters above the sinotubular junction, but distances of up to 2.0 cm have been reported. Although this type of abnormality is not of major clinical significance, it may lead to difficulty in coronary artery cannulation during angiography.
Separate Ostia of the Left Circumflex and Left Coronary Arteries
The LCx and LCA may have separate ostia at the left sinus of Valsalva. This occurs in a small percentage of people (0.4%). Although this may lead to difficulties with cannulation at angiography, it also may provide collateral pathways in the event of proximal LCA disease. This anomaly has been reported to be associated with an increased incidence of aortic valve disease.
Absent Left Circumflex Artery
An absent LCx artery occurs in less than 0.005% of the population. In this anomaly a “superdominant” right coronary artery crosses the crux of the heart and ascends in the left AV groove in retrograde fashion to supply the posterolateral and lateral wall of the left ventricle.
Origin of the Left Circumflex Artery From the Right Sinus of Valsalva or Right Coronary Artery
Origin of the LCx from the right sinus of Valsalva or the proximal RCA is one of the most common of the coronary anomalies and constitutes between 30% and 60% of anomalies identified in angiographic studies. The prevalence of this anomaly is approximately 0.4%. The anomalous LCx more commonly arises from the right sinus of Valsalva (69%) than the proximal RCA (31%) and always courses posterior to the aorta. The LCx courses posterior to the aorta before supplying the left lateral wall of the heart. It may be an isolated anomaly with the LAD originating normally from the LCMA or may be associated with other branch anomalies such as the LAD originating from the anomalous LCx artery. At angiography contrast injection into the RCA may fail to opacify an anomalous LCx, and the catheter may need to be placed in the posterior aspect or the right sinus of Valsalva to visualize the vessel. Mismanagement may result if the angiographer assumes the LCx is occluded or congenitally absent. The cardiac surgeon should be informed of the presence of this anomaly to avoid injury during valve replacement surgery. This anomaly is benign.
Anomalous Origin of the Left Main Coronary Artery or Right Coronary Artery From the Posterior Sinus of Valsalva
Anomalous origin of the LMCA or RCA from the posterior sinus of Valsalva is extremely rare. This anomaly was identified in only 5 patients (RCA in 4 patients and LCA in 1 patient) in the study by Yamanaka and Hobbs of more than 126,000 patient angiograms. This anomaly is not associated with symptoms or complications and is considered benign.
Anomalous Aortic Origin With Potentially “Malignant” Course
Single Coronary Artery
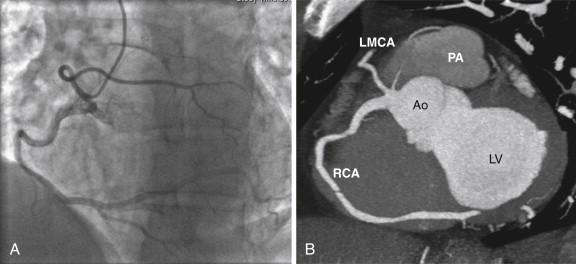
A single coronary artery is a rare occurrence (<0.05%) in which all the coronary arteries arise from a single ostium ( Fig. 37-1 ). This vessel may take the same proximal course as the normal right or left coronary artery before dividing into two branches that supply the same distributions as the right and left coronary arteries. This anomaly is associated with an increased risk for sudden death if a major coronary branch passes between the aorta and the right ventricular outflow tract. The Lipton classification scheme is used to further describe these anomalies. In group I anomalies there is a single coronary artery, which takes the course of the right or left coronary artery and supplies the entire myocardium. In group II anomalies the anomalous vessels arise from a normal proximal left or right coronary artery and take an interarterial or more commonly septal course before supplying their usual territory. In group III anomalies the LCx and LAD arise separately from the proximal RCA.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree