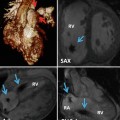
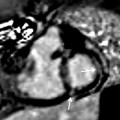
Fig. 27.1
MR techniques in the evaluation of coronary anomalies. Contrast-enhanced 3D coronary MRA (a) demonstrates right coronary artery aneurysm (arrow) which is partially filled with non-enhancing thrombus (asterisk). Late gadolinium enhancement inversion recovery image (b) demonstrates segmental infarction involving the basal and mid-inferior and inferoseptal walls (arrowhead) within the distribution supplied by the right coronary artery. Cine MR images at end diastole (c) and end systole (d) demonstrate the associated wall motion abnormality (black arrows) involving the basal and mid-inferior and inferoseptal walls
There have been several different MR methods applied for coronary MRA including both dark-blood and bright-blood techniques. Standard 2D spin-echo MR imaging had been the predominant technique used early on for visualization of the proximal coronary artery segments [22]. However, since 1991, coronary MRA has been mainly performed with “bright blood” gradient-echo techniques. Initial work focused on acquiring multiple thin 2D stacked slices gated to the cardiac cycle and processed with maximum intensity projection (MIP) algorithm to produce a coronary angiogram [23, 24]. Additional 2D methods such as selective arterial tagging with subsequent subtraction, interleaved spiral K-space scanning, and an ultrafast K-space segmented technique have also been described [20].
3D coronary MRA offers several advantages over 2D techniques. With 3D techniques, a volumetric acquisition of thin, truly contiguous partition images that encompass the complete coronary arterial tree can be acquired in 1 acquisition, minimizing the reliance on slice orientation [20]. 3D coronary MRA techniques were developed in the early 1990s, and investigators were able to successfully image volumes up to 64 mm thick across the aortic root with sufficient contrast between blood vessels and surrounding tissues. Further advancements in MRA techniques have led the development of various 3D whole-heart coronary MRA techniques with further improvements in tissue contrast for improved delineation of the coronary arteries [25–30]. Disadvantages over the 2D acquisitions are increased susceptibility to motion artifacts related to phase encoding in two directions [31, 32] and longer acquisition times. Multiple techniques have been applied to overcome the difficulties associated with coronary artery imaging, including suppression of respiratory artifacts with the use of signal averaging, breath-holding or respiratory triggering [33], suppression of cardiac motion with cardiac gating, and ultrafast imaging sequences [34] with optimization of coronary artery signal to noise ratio. Other methods to reduce imaging time include limited resolution and coverage for protocols designed for detection of coronary anomalies and not for coronary stenosis detection [35].
The most important advantage of coronary MRA is that it does not require radiation. Another advantage is that it can often be performed free-breathing, often without the need for exogenous contrast agents and beta-blockers, making it useful for diagnostic evaluation in young patients with symptoms of possible anomalous coronary arteries [17] and allowing for repeat acquisitions on the same day in case of artifacts. Additionally, adjunct MR sequences such as velocity-encoded phase-contrast imaging and cine and late gadolinium enhancement imaging can further aid in demonstrating the flow directionality, hemodynamic significance, and structural and functional consequences of anomalous vessels [36]. Limiting factors with coronary MRA include its dependence on regular heart rhythm and respiration pattern for optimizing image quality and shortening image acquisition times. There are contraindications in patients with pacemakers, internal cardiac defibrillations, intracranial surgical clips, hearing aids, or neurostimulators, and MR-compatible metallic implants or clips may lead to problematic susceptibility artifact (i.e., following coronary artery bypass surgery). In addition, because of limited spatial resolution, distal coronary segments are often not well visualized with coronary MRA [37, 38]. Thus, alternative techniques such as coronary CTA are the preferred modality for evaluation of small collateral vessels and arteriovenous fistulas [39].
Coronary CTA
Coronary CTA is a valuable noninvasive 3-dimensional modality for delineating coronary artery anomalies, particularly in patients with equivocal x-ray coronary angiography findings [40], and is recommended as an initial screening method for evaluation of individuals with possible anomalous coronary arteries as part of the differential diagnosis [16, 41]. Recently, numerous studies have confirmed that coronary CTA is superior to conventional x-ray angiography in the detection of coronary artery anomalies [42]. In one study of 1,758 patients who underwent coronary CTA, 28 patients with anomalous coronary arteries were identified. Twenty of these patients subsequently underwent conventional x-ray coronary angiography. The anomalous coronary arteries could be accurately diagnosed with conventional x-ray angiography in only 11 of these patients [42].
There have been significant advances with coronary CTA over the past decade focused on improvement of image quality and reduction of patient radiation exposure. Multi-detector CTA, particularly with newer generation scanners, permits high-speed scanning of large volumes with high in-plane and through-plane resolution with isotropic voxels for evaluation in nearly any imaging plane without loss of spatial resolution. It is important to note that available acquisition and radiation dose reduction modes vary significantly depending on the manufacturer and model of specific CT systems. In depth discussion and comparison of the available technologies will not be covered in this chapter. However, common technical issues including dose reduction features will be mentioned.
ECG gating is an essential requirement for all coronary CTA imaging. Synchronization with the ECG is generally performed with detection of the R wave on the ECG signal. The “R-R interval” represents the time between consecutive R waves. The timing of image acquisition or reconstruction is often expressed as either a percent of the R-R interval or actual delay time (e.g., “400 ms” would be 400 ms after the R wave and “-400 ms” would be 400 ms before the R wave). ECG gating can be performed either in prospective or retrospective modes depending on the type of image acquisition. Retrospective gating is often performed in conjunction with conventional spiral imaging, simultaneous recording of the ECG over several heart beats (usually 6–10 beats), and very low pitch to allow for image reconstruction at any phase of the cardiac cycle. The cardiac phase least affected by cardiac motion artifact can then be chosen for image interpretation. Prospective triggering is commonly performed in conjunction with axial (step and shoot) scanning mode, with data acquisition triggered by a pre-specified time or phase of the cardiac cycle and performed over several heart beats until the entire heart is imaged. Data acquisition can be performed at a single phase of the cardiac cycle (e.g., 70 % of the R-R interval) or across a range of phases (60–80 % of the R-R interval) sometimes denoted as “padding.” The advantage with retrospective gating is the ability to obtain ventricular functional information, since image reconstruction can be performed throughout the cardiac cycle. However, this is at the expense of increased patient radiation exposure. The advantage of prospective triggering is the significant reduction in radiation exposure, particularly when little or no padding is used [43–45]. The disadvantage with prospective triggering is the more limited dataset available based on a pre-specified chosen phase (or range of phases if padding is used) of the cardiac cycle with limited ability to compensate for an arrhythmia that may occur during acquisition.
Excellent temporal and spatial resolution is necessary for adequate visualization and interpretation of the small, moving coronary arteries. For CT scanners, temporal resolution is largely dependent on the rotation speed of the gantry, although other parameters may also be important. An estimate of temporal resolution for most scanners is simply the amount of time needed for the gantry to rotate half of a complete rotation (e.g., 150 ms for a scanner with a 300 ms gantry rotation time). Multi-cycle reconstruction techniques can significantly improve the effective temporal resolution by merging the datasets from several different heart beats but at the expense of increased radiation exposure. Dual-source scanners utilize two sets of sources and detector rows positioned at 90° angles to each other within the gantry and allow for simultaneous data acquisition and improved effective temporal resolution of the scanner to one-quarter of the gantry rotation time (e.g., 75 ms for gantry rotation of 300 ms). Given the limitations of temporal resolution with CT, patient heart rates of less than 60–65 beats per minute are often recommended to optimize image quality. Temporary reduction in heart rate is commonly achieved by the administration of beta-adrenergic blocking agents (or calcium channel blockers) which are given to the patient prior to scanning. Beta-blockers and calcium channel blockers have the added advantage of being able to suppress ectopic beats (e.g., premature ventricular contractions) that can adversely affect image quality. However, premedications should be used with caution in patients with low blood pressure or acutely ill. The spatial resolution of most scanners is 0.4–0.5 mm. Current generation CT scanners can acquire images with slice thickness as thin as 0.5 mm and generate nearly isotropic voxels as small as 0.5 mm3. Field of view of the reconstructions should be generally limited to the smallest diameter possible that includes the cardiac structures and generally no more than 20–25 cm for optimal viewing (assuming a standard 512 × 512 image reconstruction matrix), with axial slice thickness set at the thinnest slices possible. Nitrates are commonly administered prior to scan acquisition for coronary vasodilatation to optimize vessel visualization.
The administration of intravenous iodinated contrast is necessary for optimal evaluation of coronary CTA. Depending on individual manufacturers, models, and protocols, contrast rates range from 4 to 7 mL/s and total contrast volumes usually range from 50 to 120 mL. Dual-head injection pumps allow for injection of saline (40–50 mL) immediately following the contrast injection to maximize contrast enhancement of the coronary arteries. Either “bolus tracking” or the “test bolus” method with tracking of a region of interest (e.g., ascending aorta) may be used to ensure accurate timing of data acquisition with contrast enhancement of the coronary arteries.
The primary disadvantage of coronary CTA is exposure to ionizing radiation, of particular concern in younger patients. Many different strategies have been employed to reduce the radiation exposure associated with coronary CTA, most of which are individualized dependent on patient and scanner-specific qualities. Examples of operator-dependent parameters include choice of scan mode, tube voltage, and tube current dependent on patient size including automated adjustments available on some scanners and scan length. Single-beat whole-heart imaging via wide detector systems (e.g., 320-detector CT system) or using high-pitch helical mode has been shown to provide diagnostic image quality with potential to reduce radiation exposure by over 90 % [46, 47]. Post-processing techniques such as iterative reconstruction algorithms can provide improved image quality and reduced noise, allowing for diagnostic image acquisition at lower tube potential and current compared to standard filtered back projection algorithms [48–50].
Image Interpretation
Visualization of the coronary arteries from different imaging planes is often essential for accurate image interpretation. Isotropic voxels in three-dimensional volumetric datasets (MRA or CTA) allow for MPR with minimal image distortion. Most 3D software packages allow for display of three orthogonal views simultaneously, allowing for manual manipulation of the planes in order to optimize the view for each coronary artery and segment. The use of curved MPR images, often created by an automated or semiautomated algorithm, allows for display of the entire coronary vessel in a single 2-dimentional (2D) image, with the ability to rotate the artery around a focal point. Maximum intensity projections (MIP) are created using thicker sections and can be helpful to reduce image noise and/or visualize a longer vessel segment. Finally, volume-rendered 3D images allow for interpretation of structural relationships such as vessel course in relation to other cardiac or vascular structures with exclusion of surrounding structures such as bones, lung tissue, and extracardiac vessels.
Normal Coronary Artery Anatomy
Embryology
The development of a blood vascular system occurs relatively early during the gestation period, characterized by differentiation of precursor cells into primitive blood cells and endothelial cells. The first endothelial cells appear during formation of the heart tubes and form the endocardial endothelium. As the wall of the heart tube thickens and the cardiac jelly diminishes, the number of myocytes increase in number and the endocardial surface becomes progressively more trabeculated, establishing an endocardial-lined sinusoidal system which minimizes diffusion distances to the myocardium. This sinusoidal system precedes any evidence of vascularization [51].
The initial feature of a developing coronary vasculature occurs around the fourth week of embryogenesis with the appearance of vascular buds or blood islands that arise in the epicardium and consist of a layer of endothelial cells distended by nucleated erythrocytes. These blood islands appear to form from endothelial precursor cells from the liver region which migrate to the epicardium as opposed to endocardial endothelial cells [52]. The gradual disappearance of cardiac jelly and eventual compaction of the myocardium seems to correlate with the appearance of the blood islands. These blood islands initially appear near the ventricular apex in the interventricular sulcus and then eventually over other regions of the ventricles. The blood islands gradually coalesce and form the rudiments of a network of vascular channels, preferentially forming in the sulci or indentations of the epicardial surface [53]. Following early capillarization, the venous vessels appear around the sixth week and arterial vessels during the transition from sixth to seventh week of embryogenesis. Although different concepts have been put forth regarding the development of the coronary arteries from the aorta, current evidence suggests that major coronary arteries form by coalescence of microvessels which grow toward and penetrate the aorta [54], an event that occurs as early as 44 days after gestation [53]. Several studies have shown that the left coronary artery appears to establish a connection earlier than the right [55, 56]. The development of coronary arteries proceeds in orderly sequence, proximal-distal, with smooth muscle alpha actin first expressed at the site of their aortic connection regulated by neural crest [51]. Disruption of the neural crest leads to variability in the site of origin and symmetry of the coronary arteries [51]. Thickening of the media and adventitia, formation of the internal elastic lamina, and increase in collagen content occur between the fourth month and birth [57], with continued increase both medial thickness, length of the arterial segments, and numerical density of arterioles during the first year after birth [58, 59].
Definitions
The basic definition of what constitutes a normal coronary artery and the normal spectrum of coronary artery variation has been the subject of many articles. In general, most experts agree that it is normal to have two coronary arteries, the right and the left. However, some confusion arises with regard to definitions of smaller arteries such as conal (infundibular) branches, particularly when these branches demonstrate independent origin from the aorta. A frequently used definition is that any configuration occurring in >1 % of an unselected general population is considered normal or normal variant [60]. It then follows that coronary anomalies are variants of normal coronary artery anatomy that occur in <1 % of the general population.
Another basic nomenclature principle is that coronary vessels are named for the structures that they supply rather than for their origin. This nomenclature is based on the fundamental embryologic principles, namely, that the coronary vessels arise within the developing epicardium and only later attach to the aorta. Thus, various perturbations in the connections of the coronary vessels to each other and to the aorta occur subsequent to the formation of the coronary vessels in the epicardium. Moreover, three main coronary vessels (left anterior descending, circumflex, and right coronary) are considered arteries and the smaller, more distal vessels are termed coronary branches.
Normal Variants
There are several commonly recognized variations in normal coronary artery anatomy. Some of the more typical coronary artery variants are described below.
Vasculature to the Inferior Wall
The vascular supply to the inferior wall has a spectrum of variants. The origin of the posterior descending artery (PDA) and posterolateral branches that supply the inferior wall can originate from the right coronary artery (RCA) only (right dominant), the left circumflex (LCx) artery only (left dominant), or encompass multiple branches from both arteries (codominant). In most patients the PDA arises from the distal portion of the coronary artery, but some patients have early takeoff of the PDA from the RCA (split RCA), which then courses toward the apex along the diaphragmatic surface of the right ventricle. Others have a left anterior descending (LAD) artery that wraps around the apex to supply some of the apical inferior wall.
Vasculature to the Atrioventricular Node
A better definition of coronary dominance incorporates the arterial supply to the atrioventricular node, an electrically active bundle of tissue that delays conduction of impulses from the atria to the ventricles. Unfortunately, the node is not directly visualized with current coronary artery imaging techniques, but it is closely associated with central fibrous body near the apex of the Koch triangle. This triangle is enclosed by the septal leaflet of the tricuspid valve anteriorly, the coronary sinus ostium inferiorly, and the Eustachian ridge posteriorly. Typically the dominant artery that supplies the PDA and inferior wall also gives rise to small branches at the base of the heart that supply the atrioventricular node.
Vasculature to the Sinoatrial Node
The sinuatrial node is another electrically active tissue bundle that sits near the junction of the right atrium and superior vena cava and serves as the primary pacemaker of the heart. This node is supplied by a single branch from the proximal RCA in 60 % of people. Other variants include sinoatrial node branch from the proximal LCx or distal RCA or LCx artery [61].
Ramus Intermedius
Instead of bifurcating into the LAD and LCx arteries, in some patients the left main trifurcates into an LAD, LCx, and ramus intermedius artery. The ramus intermedius typically supplies the lateral and inferior walls in a territory similar to that supplied by the diagonal or obtuse marginal branches. As a result, the branches that would normally supply these regions are either diminutive or nonexistent and have coalesced into the ramus intermedius.
Right Superior Septal Perforator
The right superior septal perforator is a normal variant seen in approximately 3 % of patients [62]. This branch supplies the anterior septum in a similar territory to other septal perforators but arises from the proximal RCA or right sinus of Valsalva instead of the LAD.
Multiple Coronary Ostia
In addition to the typical LCA and RCA origins from the aorta, some patients have smaller branches that arise directly from the aorta as opposed to from the coronary arteries. Common variants of this type include direct origin of the conus or sinoatrial node branch from the aorta (Fig. 27.2). While these variants may have clinical consequences if unrecognized during surgery or coronary catheterization, for the majority of people, these are not clinically significant.
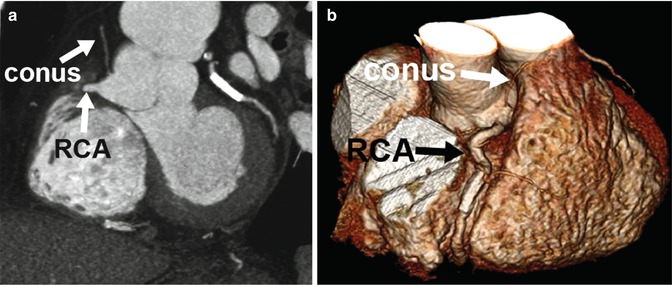

Fig. 27.2
Normal variant of multiple coronary ostia. Coronary CTA 2D thin maximum intensity projection (a) and 3D volume-rendered (b) images demonstrating a common coronary artery variant of separate origin of the conus branch directly from the aortic root instead of from the right coronary artery (RCA)
Myocardial Bridging
Myocardial bridging refers to the intramyocardial course of a portion of a normally positioned epicardial coronary artery (Fig. 27.3). The degree in which the coronary artery “dives” into the myocardium is variable in depth and length. With the advent of coronary CTA and MRA, allowing visualization of extra-coronary structures, this variant has been noted to occur much more frequently than previously reported by x-ray coronary angiography. Although typically seen in the mid-LAD segment, myocardial bridges can occur in any coronary artery segment. Although systolic narrowing may be visualized on coronary artery imaging, these findings are rarely clinically significant since most coronary blood flow occurs in diastole.
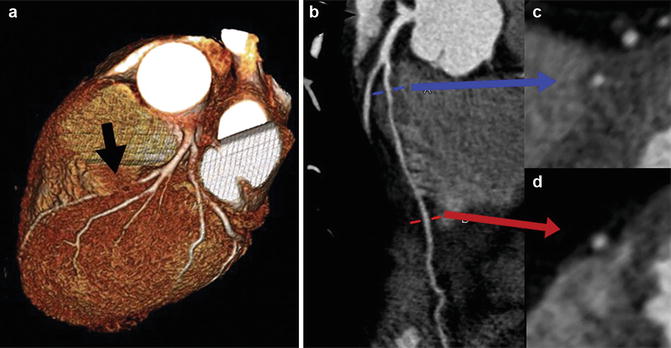

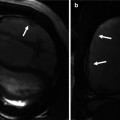
Fig. 27.3
Normal variant of myocardial bridging. Coronary CTA 3D volume-rendered image (a) demonstrates that a portion of the left anterior descending artery (LAD) is obscured by overlying myocardium (black arrow). Curved multiplanar reformatted images of the LAD (b) and corresponding multiplanar reformatted images axial to the vessel (c, d) demonstrate intramyocardial bridge with the mid-segment of LAD surrounded by myocardium (blue arrow, image c) and normal epicardial position of the distal LAD surrounded by epicardial fat (red arrow, image d)
Coronary Anomalies: Clinical Implications
Incidence
Depending on the series, isolated congenital coronary artery anomalies have been reported in approximately 1.3 % (range 0.2–5.6 %) of patients undergoing x-ray coronary angiography [1–4]. Variations in number are likely related to referral bias in some centers not reflective of the general population and variability in definitions of anomalous versus normal variant coronaries. Although the aortic root is normal in most patients with coronary anomalies, about 26 % of coronary anomalies involve some type of aortic root abnormality, such as asymmetry of the aortic sinuses [63]. In patients with more substantial cardiac defects of the outflow tracts, such as transposition of the great arteries, increased variation of the coronary artery pattern is noted, and it becomes important when describing these patterns to relate them specifically to the aortic and pulmonic valves [64]. The most common coronary anomaly is absent left main with separate origin of the LAD and LCx arteries from the left sinus of Valsalva, with an incidence of 0.41 % followed by the LCx arising from the RCA or directly from the right sinus of Valsalva, with an incidence of 0.37 % [65]. Both of these anomalies are considered benign in clinical significance. The most common potentially serious coronary artery anomaly is anomalous origin of the RCA from the left sinus of Valsalva, with incidence of 0.107 % [65] (Table 27.1).
Table 27.1
Incidence of specific coronary anomalies
Number | Incidence(%) | Anomalies(%) | |
|---|---|---|---|
Benign | |||
Absent left main | 513 | 0.41 | 30.4 |
LCX from right sinus or RCA | 467 | 0.37 | 27.7 |
Coronary artery from noncoronary sinus | |||
1. LCA from noncoronary sinus | 1 | 0.0008 | 0.06 |
2. RCA from noncoronary sinus | 4 | 0.003 | 0.24 |
Anomalous origin from above the sinuses | |||
1. LCA from ascending aorta | 16 | 0.013 | 0.95 |
2. RCA from ascending aorta | 188 | 0.15 | 11.2 |
Small coronary artery fistulae | 163 | 0.12 | 9.7 |
Potentially serious | |||
Coronary artery from pulmonary artery | |||
1. LM from pulmonary artery | 10 | 0.008 | 0.59 |
2. LAD from pulmonary artery | 1 | 0.0008 | 0.06 |
3. RCA from pulmonary artery | 2 | 0.002 | 0.12 |
Coronary artery from opposite sinus | |||
1. LM from right sinus | 22 | 0.017 | 1.3 |
2. LAD from right sinus | 38 | 0.03 | 2.3 |
3. RCA form left sinus | 136 | 0.107 | 8.1 |
Single coronaryartery | 56 | 0.044 | 3.3 |
1. R-I | 1 | 0.0008 | 0.06 |
2. R-II | 19 | 0.015 | 1.1 |
3. R-III | 5 | 0.004 | 0.3 |
4. L-I | 20 | 0.016 | 1.2 |
5. L-II | 11 | 0.009 | 0.65 |
Multiple or large-sized fistulae | 62 | 0.06 | 3.7 |
Clinical Presentation
Many studies have demonstrated that most coronary artery anomalies are discovered as benign, incidental findings upon angiography studies. Some of these “benign” anomalies include (1) absent left main, (2) ectopic origin of the LCx artery from the right sinus of Valsalva, (3) ectopic coronary origin from the posterior sinus of Valsalva, (4) anomalous coronary origin from the ascending aorta, (5) absent circumflex, (6) intercoronary communications, and (7) small coronary artery fistula. Other anomalies may be associated with potentially more serious sequelae such as angina pectoris, myocardial infarction, syncope, cardiac arrhythmias, congestive heart failure, or even sudden death. Potentially serious anomalies include (1) ectopic coronary origin from the pulmonary artery, (2) ectopic coronary origin from the opposite sinus of Valsalva, (3) single coronary artery, and (4) large coronary fistula [1]. The etiology of myocardial ischemia may be related to disturbed kinetics from oblique origin, ostial stenosis, compression of intramural course, loss of reservoir capacity, and increased myocardial oxygen demand associated with exercise. Physical examination findings depend on the coronary anomaly and are most often entirely normal.
Classification Schemes
Different classification criteria have been described for coronary artery anomalies. Some investigators prefer to categorize anomalies based solely on clinical significance, such as benign, relevant, severe, or critical [66] (Table 27.2). Other schemes focus on first assessing anatomic variation independent from clinical and hemodynamic sequelae, with judgment about clinical relevance as a secondary clinical classification. Anatomic schemes commonly divide coronary artery anomalies into three general classifications. These include anomalies of origin and course, anomalies of intrinsic coronary arterial anatomy, and anomalies of coronary termination (Table 27.3). Coronary anomalies of origin and course have the highest risk of developing major cardiac events.
Table 27.2
Clinical significance-based classification of coronary artery anomalies
Class | Coronary artery anomaly |
|---|---|
I. Benign | Ectopic origin of LCX from right sinus |
Ectopic origin of LCX from the RCA | |
Separate origin of LCX and LAD | |
Duplicated LAD | |
II. Relevant | Coronary artery fistula |
Related to myocardial ischemia | Single coronary artery R-L, I-II-III, A-P |
Ectopic origin of LCA from PA | |
Coronary artery atresia | |
Hypoplastic coronary artery | |
III. Severe | Ectopic origin of LCA from the right sinus |
Potentially related to sudden death | Ectopic origin of RCA from the left sinus |
Ectopic origin of RCA from the PA | |
Single coronary artery R-L, I-II-III, B | |
IV. Critical | Class II and superimposed CAD |
Related to sudden death/myocardial ischemia and associated with superimposed CAD | Class III and superimposed CAD |
Table 27.3
Anatomic classification of coronary artery anomalies
Anomalies of origin and course |
Absent left main |
Anomalous coronary ostium outside the aortic sinuses |
High takeoff |
Anomalous origin of coronary artery from pulmonary arterya |
Anomalous coronary ostium at an improper sinus with anomalous course |
Anterior |
Interarteriala |
Intraseptal |
Posterior |
Single coronary artery |
Anomalies of intrinsic anatomy |
Congenital ostial stenosis or atresia |
Coronary ectasia and aneurysm |
Duplicated coronary arteries |
Subendocardial coronary course |
Coronary crossing |
Anomalies of termination |
Coronary artery fistulasa |
Extracardiac terminations |
Coronary arcade |
Isolated Coronary Anomalies
Anomalies of Origin and Course
The majority of clinically significant coronary artery anomalies are within this group of anomalies. There are three main subcategories within this group including (A) absent left main (LM) artery, (B) anomalous ostium outside the aortic sinuses, and (C) anomalous ostium at an improper sinus.
Absent Left Main
Absent left main, also known as separate origin of LAD and LCX, is typically a benign configuration where the LAD and LCX each have well-defined, separate ostia from the left sinus of Valsalva (Fig. 27.4). Although inability to separately cannulate the LAD and LCX at x-ray angiography may lead to misinterpretation that one of these arteries is completely occluded, with potential failure to perfuse both vessels during cardiopulmonary bypass [67], the wide field of view and tissue characterization possible with coronary CTA and MRA make this diagnostic error uncommon [68]. There is an increased incidence of left coronary artery dominance and myocardial bridging. However, there is no increased association with congenital heart anomalies or coronary artery disease [67, 69].
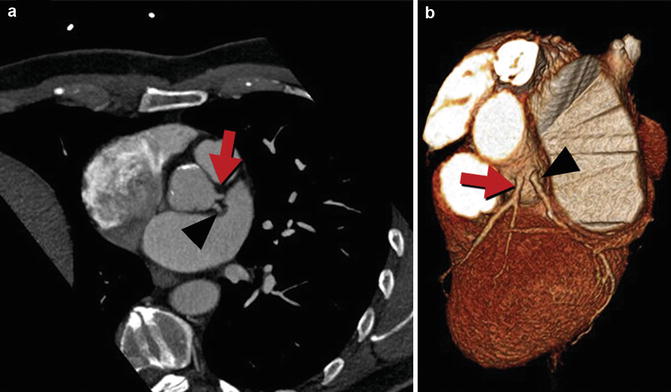

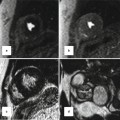
Fig. 27.4
Absent left main or separate origin of the left anterior descending (LAD) and circumflex arteries. Coronary CTA multiplanar reformatted image (a) and corresponding 3D volume-rendered image (b) demonstrate that the LAD (red arrow) and circumflex (black arrowhead) arteries arise directly from the left coronary sinus without a common ostium (absent left main)
Anomalous Coronary Ostium Outside the Aortic Sinuses
There is a large subset of coronary anomalies, including origin of the left coronary artery from the pulmonary artery (ALCAPA) (Fig. 27.5), that are part of this subcategory. ALCAPA is also known by the eponym of Bland-White-Garland syndrome with estimated incidence of 0.008 % [65]. This anomaly most often presents as an isolated defect, but in 5 % of cases may be associated with other congenital heart defects, including atrial septal defect, ventricular septal defect, and aortic coarctation. Symptoms usually present in infants or children with symptoms of myocardial ischemia due to coronary steal phenomenon or congestive heart failure. Left to right shunting from the higher pressure left coronary arterial system to the lower pressure arterial system occurs via RCA to left coronary artery (LCA) collateral vessels, with retrograde flow in the left coronary arterial circuit. Thus, blood flows from the aorta –> RCA –> collateral vessels –> LCA –> low pressure pulmonary circuit. The resultant circulatory insufficiency results in left ventricular dysfunction, myocardial infarction, or life-threatening cardiac arrhythmias.
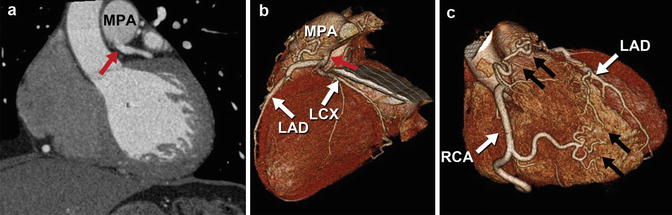

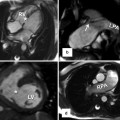
Fig. 27.5
Anomalous left coronary artery from the pulmonary artery (ALCAPA). Coronary CTA multiplanar reformatted images (a) and corresponding 3D volume-rendered images (b, c) demonstrate anomalous origin of the left coronary artery from the main pulmonary artery (MPA) (red arrow). The right coronary artery (RCA) demonstrates normal origin from the right aortic sinus and is significantly dilated due to the inherent left to right shunting present at the coronary artery level with associated large collateral vessels (black arrows) between the right and left coronary artery systems. LAD left anterior descending artery, LCX left circumflex artery
ALCAPA is associated with 90 % mortality if not surgically corrected [70]. Although rare, there have been numerous reports of this anomaly presenting in the adult, with clinical presentations of heart failure from chronic mitral insufficiency or global ischemic cardiomyopathy [71]. There is still ongoing risk of sudden cardiac death due to ischemic malignant ventricular arrhythmias [72]. Survival beyond infancy is attributed to the development of abundant intercoronary collateral arteries, an alteration in hemodynamics that encourages antegrade blood flow in the left coronary arterial tree, and reduction in the size of the left ventricular myocardium supplied by the LCA [70]. Once the diagnosis of ALCAPA is made, early surgical repair is recommended to prevent future potential complications. Surgical repair procedures include aortocoronary saphenous vein or internal mammary artery bypass grafting, aortic root reimplantation with or without a pulmonary flap, or intrapulmonary baffling [72]. Other coronary anomalies arising from the pulmonary artery are even more rare, including anomalous LAD from the pulmonary artery with estimated incidence of 0.0008 % and anomalous RCA from the pulmonary artery (ARCAPA) with estimated incidence of 0.002 % [65] (Fig. 27.6).
Get Clinical Tree app for offline access

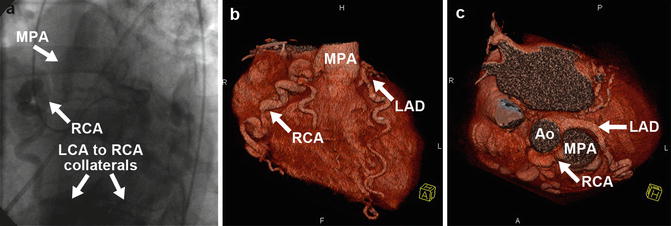
Fig. 27.6
Anomalous right coronary artery from the pulmonary artery (ARCAPA). X-ray coronary angiogram (a) and 3D coronary CTA volume-rendered images (b, c) demonstrating anomalous origin of the right coronary artery (RCA




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree