Coronary artery disease is the leading cause of death in the United States. Surgical revascularization for coronary artery disease (CAD) with coronary artery bypass graft (CABG) surgery is the recommended treatment for patients with significant left main disease, severe (≥70%) proximal left anterior descending (LAD) stenosis, significant two-vessel disease with involvement of the proximal LAD, and severe three-vessel CAD. The aim of CABG is to restore myocardial perfusion to the hypoperfused ischemic territories, which not only relieves angina and improves exercise tolerance but more importantly results in improved long-term survival.
Despite its obvious benefit, CABG over time may result in complications, particularly those of graft stenosis and occlusion, as well as progression of atherosclerotic disease in the native grafted and nongrafted coronary arteries. Saphenous venous grafts (SVGs) have a higher occlusion rate than internal mammary artery (IMA) grafts. The 1-year patency rates of IMA grafts and SVGs are not significantly different. However, 5 and 10 years after revascularization, patency rates for IMA grafts are superior at 88% and 83%, respectively, compared to 73% and 41% for SVGs. Invasive coronary angiography is considered the gold standard for evaluating CABGs and native coronary arteries. The risk for major complications is less than 1% for invasive coronary angiography. However, in postoperative CABG patients who have diffusely diseased coronary arteries, varying coronary and systemic atherosclerotic disease, and degenerated grafts, invasive coronary angiography is a complicated procedure that can result in life-threatening arrhythmias, damage to the vessel wall, and significant mortality in certain patient groups.
Given the high burden of CAD and greater than 470,000 CABGs performed annually in the United States, there is a need for an alternative accurate, reliable, relatively safe, and cheaper noninvasive option for evaluating patients after CABG. In the last decade rapidly evolving multidetector computed tomography (MDCT) technology, with significantly improved spatial and temporal resolution and near-isotropic imaging with submillimeter section thickness in a single breath hold, has resulted in easy, reliable, and accurate evaluation of bypass graft patency. Computed tomography (CT) has therefore emerged as a viable noninvasive imaging modality for evaluation of bypass grafts, especially with 64-detector and higher-generation electrocardiogram-gated MDCT scanners.
Bypass grafts are less challenging to evaluate on CT than native coronary arteries because they are less affected by cardiac motion, have a wider luminal diameter, and have less calcified plaque when compared to native coronary arteries. However, evaluation of the distal anastomotic site, distal runoff vessel, and heavily calcified, small-caliber nongrafted native coronary arteries can be a significant problem resulting in limited clinical utility of CT in some patients. Progressive advances in CT technology will probably overcome this problem and allow improved evaluation of these vessels.
A sensitivity of 85% to 100% and specificity of 89% to 100% for bypass graft occlusion and significant stenosis with 64-slice CT has been reported in a number of publications. A meta-analysis published by Hamon et al documented a pooled sensitivity of 98% and specificity of 97% for stenosis greater than 50% using 64-slice MDCT coronary angiography. Although the diagnostic accuracy for evaluation of bypass grafts is high with 64-slice CT, the diagnostic accuracy for detecting significant stenosis in native vessels following revascularization is lower than in native vessels of patients who have not undergone surgery. This is because the native coronary arteries in post-CABG patients are significantly calcified, are often of smaller caliber, and are prone to increased plaque burden. With 64-slice CT the sensitivity and specificity for significant stenosis in native and distal runoff vessels following CABG ranges from 77% to 97% and 76% to 100%, respectively. High diagnostic accuracy is reported by de Graaf et al for evaluation of graft stenosis and occlusion with a 320-detector scanner. Weustink et al report an even higher diagnostic accuracy (100%) for evaluation of not only the grafts but also native coronary arteries with dual-source CT, probably because of superior temporal resolution however, the patient population in this study was about 10 years younger and had revascularization surgery less than 10 years earlier.
For evaluation of bypass grafts with CT it is important to have an understanding of CABG surgical techniques and surgical conduits, specifically bypass graft anatomy and appearance of normal grafts, as well as graft complications. CT can also guide surgical approach if reoperation is being considered.
Multidetector Computed Tomography Technique
A slow and steady heart rate ensures a diagnostic-quality CT scan. In patients with heart rates above 65 beats/minute, beta-blockers are administered orally or intravenously (e.g., metoprolol 100 mg orally or up to 25 mg intravenously) with the aim of achieving a heart rate of 65 beats/minute or less to minimize cardiac motion artifacts. As with invasive coronary angiography, 0.4 mg of sublingual nitroglycerin spray is often used just before scan acquisition, barring contraindications.
An 18-gauge angiocatheter in the right antecubital vein is preferred for contrast administration because significant streak artifact can result from contrast coursing through the left subclavian vein to the superior vena cava via the left brachiocephalic vein, which can limit evaluation of a left internal mammary artery (LIMA) graft or high SVG.
Scan delay can be determined by performing a timing bolus with 15 mL of nonionic high-concentration low- or iso-osmolar iodinated contrast medium at 5 to 7 mL/second followed by 20 mL saline flush at the same rate, with the region of interest in the aortic root at the left main coronary artery origin. Alternatively, automated bolus triggering can be applied, with scan acquisition beginning 5 to 10 seconds following achievement of a predefined threshold (usually 100 Hounsfield units [HU]) in the ascending or descending thoracic aorta.
An 80- to 100-mL volume of high-concentration, low- or iso-osmolar contrast agent is administered at 5- to 7-mL/second using a power injector to achieve adequate enhancement (>300 HU) of the coronary arterial lumen, followed by 50 mL of saline at the same flow rate. Higher flow rates are used in obese patients. Some centers calculate the amount of contrast to be used by the flow rate and contrast injection time.
Details of the prior surgical procedure are extremely useful in determining the z-axis coverage. Evaluation of the SVG should extend from the cephalad ascending aorta to the base of the heart, whereas imaging of the internal mammary graft begins at the lung apices to include the IMA origin from the subclavian artery and extends caudally to the cardiac apex. The scan is then acquired in a single breath hold from cranial-to-caudad direction during mid-to-end inspiration after initial hyperventilation to reduce heterogeneity of contrast in the right atrium, which occurs from inflow of unopacified blood from the inferior vena cava. Some centers use a caudal-to-cranial direction to minimize artifact from diaphragmatic motion.
Depending on the vendor and availability of the scanner, electrocardiogram gating is performed using prospective triggering or high-pitch (flash) acquisitions for patients with stable heart rates of less than 65 beats/minute to minimize radiation dose, and retrospective gating is reserved for patients with variable or higher than 65 beats/minute heart rates or if functional information is desired. Axial slices of the heart are acquired at 0.5- to 7-mm collimation and reconstructed at 0.5- to 1-mm collimation. For retrospectively gated scans, images are reconstructed at 60% to 80% R-R interval. The raw data are transferred to an independent offline workstation with dedicated cardiac software for review.
The axial source images are evaluated first. All data are then visualized and analyzed using a number of reconstruction techniques: (1) multiplanar reformations, (2) maximum intensity projections, (3) curved multiplanar reconstructions, (4) linear lumen view, and (5) three-dimensional (3D) volume rendering (VR).
Each bypass graft is evaluated in turn for patency, stenosis, or occlusion with specific analysis of the (1) proximal anastomosis, (2) graft proper, (3) distal anastomosis, (4) distal runoff vessel, and (5) proximity of graft to the sternum or anterior chest wall. The native coronary arteries are also evaluated for stenosis.
Three dimensional VR images exquisitely depict the complex surgical anatomy of the bypass grafts, including the proximal and distal anastomosis and cardiac topography.
Coronary graft assessment broadly includes anatomy, patency, complications, and relationship to chest wall.
Anatomy of Bypass Graft Conduits
Saphenous Venous Grafts
The first CABG in the early 1960s was performed with an SVG. SVGs are readily available, easy to harvest from the lower extremities, and less prone to vasospasm. However, they are prone to intimal hyperplasia when exposed to systemic blood pressure because of lack of nitric oxide and develop atherosclerosis over time that results in poor long-term patency rates. In a large angiographic follow-up study of 5065 patients after CABG, 88% of grafts were patent perioperatively, 81% at 1 year, 75% at 5 years, and only 50% at 15 years. However, with continuing improvements in surgical techniques along with the concomitant use of antiplatelet or anticoagulant therapy and lipid-lowering drugs, SVGs remain an important and frequently used conduit because of improved patency rates.
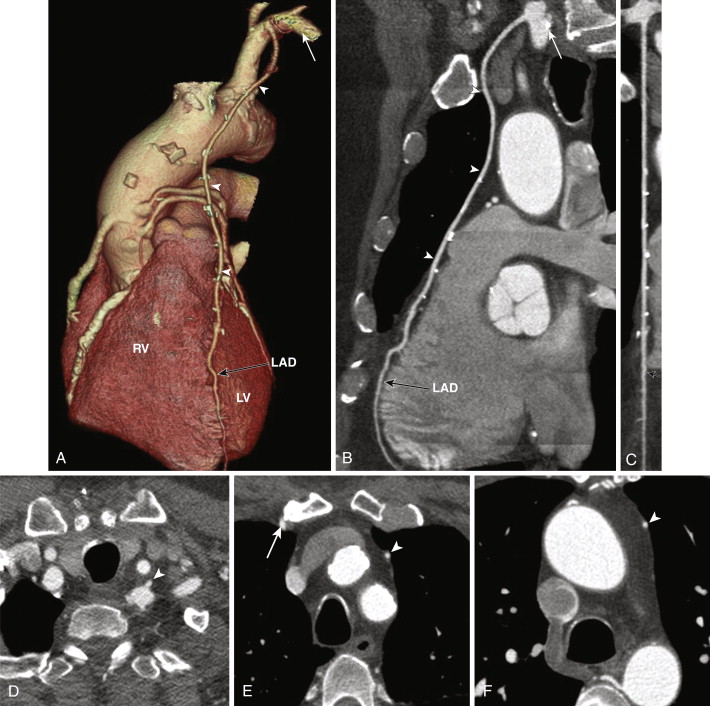
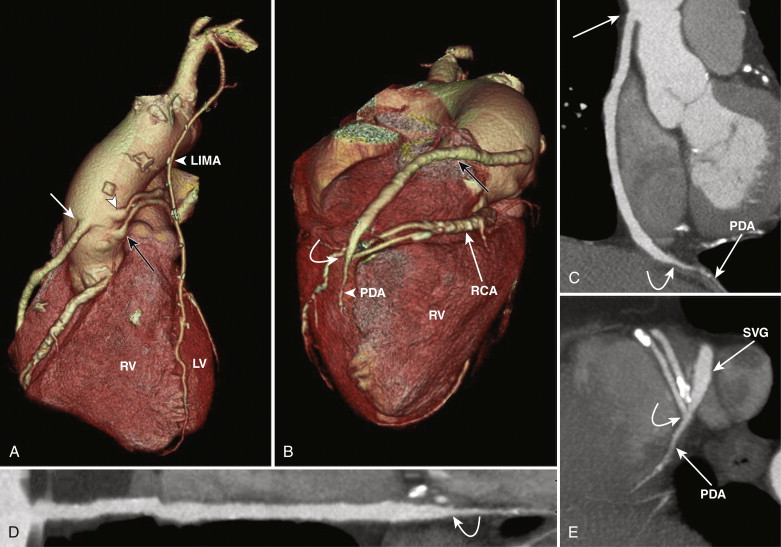
SVGs are attached proximally on the anterior wall of the ascending aorta and are grafted distally to the coronary artery beyond the significant stenosis or obstruction ( Figs. 39-1 and 39-2 ). For revascularization of the right coronary artery (RCA), posterior descending artery (PDA), or distal LAD artery, SVG are anastomosed on the right side of the anterior ascending aorta proximally, either by being directly sutured to the aorta or by means of an aortovenous connector device that allows a quick, sutureless attachment without the need to clamp target vessels. The distal portion of the graft often lies on the diaphragmatic surface of the heart (see Fig. 39-1 ).
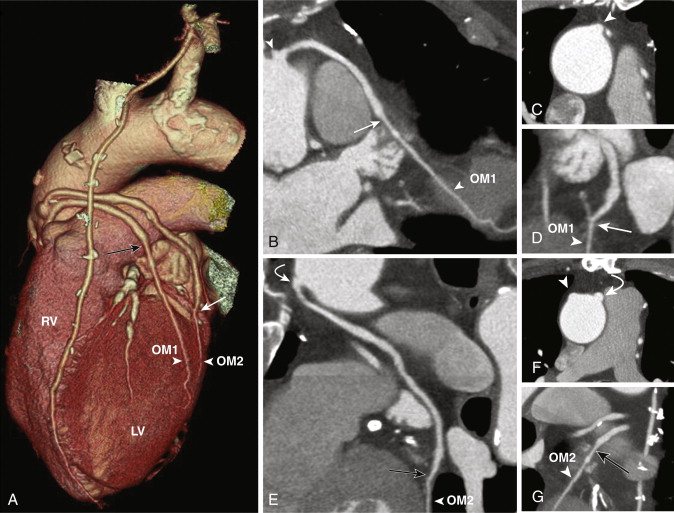
For revascularization of the anterior and lateral myocardium, the proximal anastomosis is made on the left side of the anterior ascending aorta and stabilized on the main pulmonary artery and the distal anastomosis to the mid-distal LAD, diagonal, ramus intermedius, left circumflex, or obtuse marginal arteries, beyond the significant stenosis or obstruction (see Fig. 39-2 ). SVGs can be used for sequential grafting to two or more vessels, when a side-to-side anastomosis is performed to the proximal vessel and an end-to-side anastomosis to the distal vessel.
Venous grafts are larger than arterial grafts and are less prone to streak artifact from less use of surgical clips, hence are easily evaluated on CT when compared to arterial grafts. However, the distal anastomosis may sometimes be problematic to evaluate as a larger-caliber SVG anastomoses to a significantly smaller distal runoff vessel.
Arterial Grafts
Internal Mammary Grafts
IMA grafts have a significantly higher patency rate of greater than 90%, making them the graft of choice for revascularization of obstructed coronary arteries. The presence of endothelial prostacyclin and nitric oxides in arterial grafts leads to less vasospasm. Arterial grafts are better adapted to arterial pressures and have a lower incidence of intimal hyperplasia from the presence of nonfenestrated internal elastic lamina.
Left Internal Mammary Artery
The left internal mammary artery (LIMA) is considered ideal for revascularization of the LAD and diagonal branches because of its location where it is used in situ, remaining connected at its origin at the left subclavian artery, and the mobilized distal portion is anastomosed to the LAD downstream to the critical occlusion. The LIMA can be used as a sequential graft with an end-to-side and end-to-end anastomosis to the LAD and diagonals, or both anastomoses on the LAD, one more distal than the other. The LIMA may also be used as a free graft. On CT the LIMA following its origin in the anterior inferior aspect of the proximal left subclavian artery is seen on the left side of the mediastinum, coursing down toward the anterior interventricular groove along the left heart border to anastomose to the LAD ( Fig. 39-3 ). Multiple surgical clips are typically used to ligate collateral vessels throughout its course, which can result in streak artifacts from beam hardening and can limit evaluation of the LIMA. This is overcome to a certain degree with newer scan technology such as high-definition scanners and dual-energy scanners.