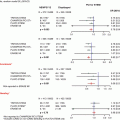
Fig. 22.1
Functionally, coronary bifurcation lesions are defined as angiographically significant lesions which involve a branch point or the immediate vicinity of a branch point between two coronary arteries larger than 2 mm in diameter
High complexity and lesion variability, high rates of restenosis and thrombosis, and a myriad of approaches to treating bifurcation lesions have left the field with many unanswered questions. Small trials, case series, and registries have reported on specialized techniques including dedicated bifurcation-specific stents, but for now interventional cardiologists are left choosing an approach based on personal preference and anecdotal experience rather than rigorous randomized data. Ongoing randomized trials with dedicated stent platforms [7] for treating bifurcation disease may ultimately provide the needed data to guide appropriate therapies, but clearly there remains significant knowledge deficit in the management of these challenging lesions.
Classification
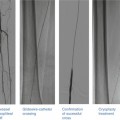
There are multiple classification systems, which can be used to characterize bifurcation lesions (Fig. 22.2). One of the more complex systems is the Lefevre system [8], which attempts to characterize the lesions by the location of the stenosis and angulation of the lesion (Fig. 22.3). Although descriptive, it is rather cumbersome to use for day-to-day practice and consensus societies such as the European Bifurcation Club (EBC) have proposed using the simpler Medina classification system [1]. With the Medina classification system, lesions can be easily characterized using a three-number designation system where the first number represents the presence or absence of disease in the proximal main branch, the second number represents the presence or absence of disease in the distal main branch, and the third number represents the presence or absence of disease in the side branch (Fig. 22.4). For each of the three locations, a zero (0) is designated for less than 50 % stenosis, and a one (1) is designated for a greater than or equal to a 50 % stenosis. Each of the three numbers is then listed in order separated by a comma to create a classification schema for the lesion. For example, a bifurcation lesion, which spares the proximal main branch but involves the distal main branch as well as the side branch, would be designated [0,1,1]. In practice, the Medina system is both easy to calculate and has been shown to have excellent inter-observer agreement for classification of lesion subtypes [9].
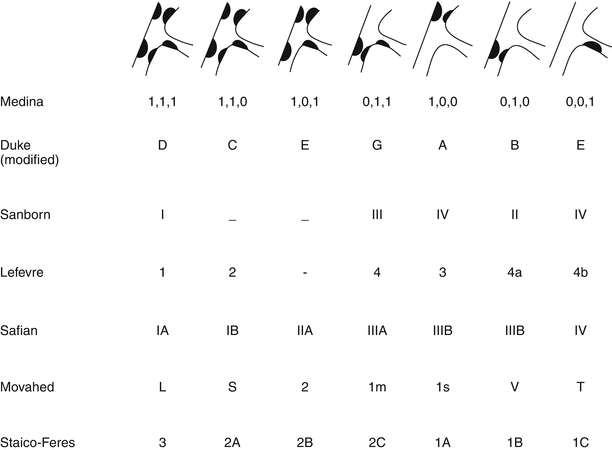
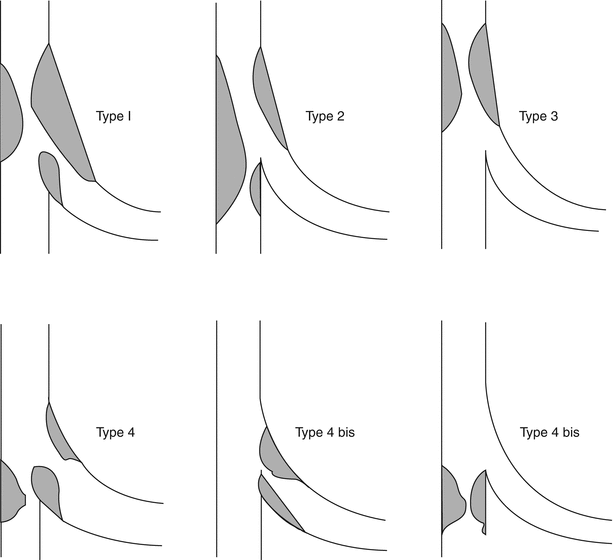
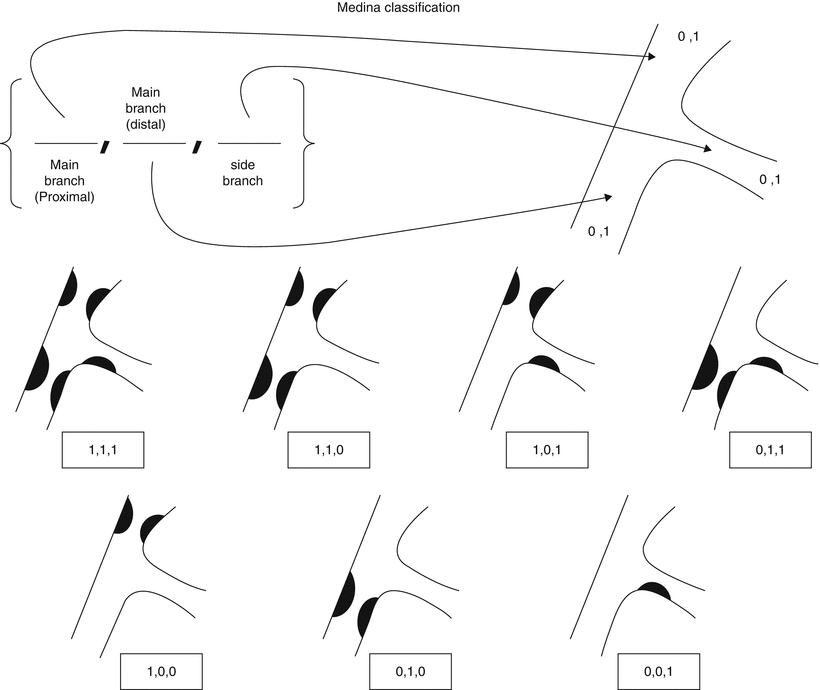

Fig. 22.2
Multiple classification systems can be used to characterize bifurcation lesions

Fig. 22.3
The Lefevre system attempts to characterize the lesions by the location of the stenosis and angulation of the lesion

Fig. 22.4
Medina classification system for bifurcation lesions
Despite its strengths, the Medina system has several weaknesses, including that it does not account for the side branch angle or side branch size, two factors which may impact an interventional approach and the number of stents which are utilized [10]. Additionally, it does not quantify the percent stenosis, but uses a binary “present or not present” approach, which can lead to the same classification for a 50 % stenosis as a 99 % stenosis in a main vessel or side branch, despite the difference in interventional risks which may be present with these disparate lesions. Nevertheless, the trade-off in ease of use may be justified, especially when considering that complex lesion subsets are easily identified with the Medina classification despite its simplistic formulation [11, 12].
Current Treatment Options for Bifurcation Lesions
When considering intervention on a bifurcation lesion, there are two general tactics that can be employed. The more conservative, or provisional, approach involves the intent to only use one stent to treat the stenosis (typically the main branch is stented). Frequently, balloon angioplasty is used to “bail out” the side branch using a “kissing balloon” inflation, which involves simultaneous inflation of two balloons in the coronary which are touching (“kissing”), with one in the main branch and one in the side branch. The operator can elect to use a second stent, but usually this would only be performed if there was significant compromise to the side branch (residual high-grade stenosis, dissection, or reduced distal flow) which could not be rectified with additional balloon angioplasty. The goal of the provisional approach is to minimize the complexity of the procedure, reduce the fluoroscopic time and contrast volume required, and reduce the resource (stent) utilization. In contradistinction to a provisional approach, a dedicated approach implies the planned use of two stents, one in the main branch and one in the side branch.
In choosing a dedicated approach, a number of techniques can be used to address the main branch and side branch plaque adequately with stents. The most straightforward two-stent technique is the T-stent [5]. In this approach, the side branch stent is deployed just across the ostium of the vessel, and the main branch stent is then deployed across the side branch to complete the “T.” It is best suited for side branches which have a 90-degree angle of intersection with the main branch. Typically, the side branch stent is deployed first, at which point the guidewire and stent-delivery equipment is then removed from the branch (to avoid jailing). Next, the main vessel stent is positioned and deployed, after which the side branch can be recrossed with a guidewire and final kissing balloon angioplasty can be performed. Modifications of this technique exist which dictate inflation of a balloon in the main vessel at the time of deployment of the side branch stent, which allows the side branch stent to be retracted back against the main vessel balloon at the time of deployment to ensure coverage of the true ostium [2, 5, 13]. There are various comparative studies of the T-technique, but a more recent comparison of T-stenting and crush stenting (discussed below) demonstrated that crush stenting had significantly less restenosis in the side branch than T-stenting, provided a final kissing balloon inflation was performed [13]. Interestingly, there was no evidence that final kissing balloon angioplasty improved restenosis in the side branch of T-stents, and overall, T-stenting had lower overall target-lesion revascularization-free survival out to 1 year when compared to crush stenting [13].
Crush stenting is another dedicated approach to dealing with complex coronary bifurcation lesions [14]. In a crush, the side branch stent is deployed so that the proximal end of the stent extends into the main vessel 3–5 mm beyond the ostium of the side branch alongside the un-deployed main vessel stent. The side branch stent is then deployed, and the guidewire and delivery system is removed. Next, the main vessel stent is deployed, which effectively “crushes” the portion of the side branch stent which extended into the main vessel against the vessel wall. In effect, this results in three layers of stent on one side of the vessel just proximal to the side branch carina and one layer on the opposite side. A guidewire is then used to recross into the side branch and final kissing angioplasty is performed with a balloon in the side branch and the main branch inflated simultaneously. Final kissing angioplasty has been demonstrated to be particularly important in crush stenting to lower the risk of restenosis at the side branch [15, 16]. Downsides to using the crush technique include the need for larger guiding catheters which can accommodate two stents simultaneously (although modified crush techniques can be performed with a 6Fr guide) and the occasional difficulty of recrossing the side branch through the multiple layers of stent. With experience, the crush technique can be performed quickly and effectively, and long-term restenosis rates are acceptable when using DES [17].
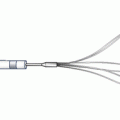
Another dedicated stent strategy is the double barrel or simultaneous kissing stent (SKS) technique. This involves the use of two stents positioned side by side in the main branch, with the proximal edge of both stents aligned (Fig. 22.5) [18]. The side branch stent extends into the side branch to cover the ostium and extends beyond any stenosis in the proximal side branch, while the main branch stent continues past the side branch origin and extends further down the main vessel. Importantly, this technique requires a guiding catheter large enough to accommodate two stents simultaneously (usually 7Fr or greater). Once both stents have been positioned and care has been given to making certain the proximal edges are lined up, both stents are simultaneously inflated and deployed at the same pressures. The difference between an SKS and a crush, which often positions the stents in the same manner, is the way the stents are deployed. In a crush, the stents are deployed sequentially, whereas in an SKS, they are deployed simultaneously. Essentially, simultaneous deployment creates a new carina which extends from the side branch to the proximal extent of the stented main branch. If one were to look down the main vessel, the previous single lumen would be converted into two lumens side by side, each outlined with stent. Because of the similarity to a shotgun barrel when visualized in this manner, this technique is also referred to as the double barrel technique. Advantages include the ability to maintain guidewire control of both the side branch and the main vessel at all times during the intervention (no need to recross). Additionally, this technique can be performed quickly in patients with compromised hemodynamics, allowing rapid reperfusion of threatened coronary distributions. However, dealing with restenosis at a later date can be an issue, as recrossing the double lumen cleanly with a guidewire can be tricky, as the guidewire often inadvertently crosses into the carina between the two stents (which does not allow passage of equipment or disrupts the architecture if the equipment crosses). Occasionally, if the vessel cannot be cleanly recrossed, the initial SKS can be converted to a crush, where either the main vessel or side vessel stent is crushed to the side and then recrossed. Overall, SKS’s main advantage is that it allows for rapid stent deployment with favorable intermediate outcomes when compared to the provisional approach [18].
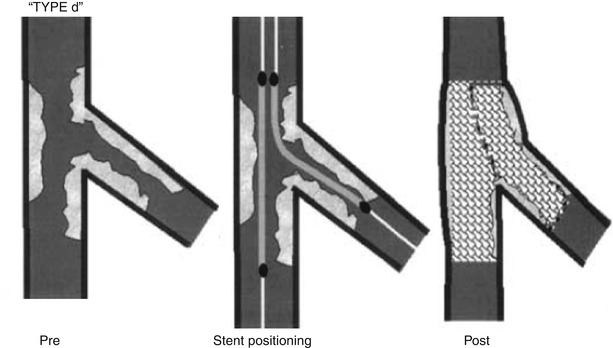

Fig. 22.5
The double barrel or simultaneous kissing stent technique
V-stenting is another method of dedicated stenting for bifurcation lesions. In this technique, which is best suited for moderate to narrowly angulated lesions, two stents are aligned with the proximal edges just proximal to the side branch and both stents are deployed together simultaneously (Fig. 22.6). The difference between V-stenting and double barrel stenting is that in the former, there is minimal to no overlap of the proximal stent portions, whereas in the latter, there is formation of a length new carina between the stents. V-stenting can be performed quickly and relatively easily, with no need for recrossing, but is really ideally suited to bifurcation lesions that have the bulk of the disease at or beyond the bifurcation (Medina classification 0, x, x).

Fig. 22.6
V-stenting is another method of dedicated stenting for bifurcation lesions
Culotte (or “Y”) stenting is a dedicated approach for bifurcation lesions. First described in 1998 by Chevalier [19], this technique takes its name from the French word for trousers. Much like a trouser leg, in this technique two stent “legs” are extended down the main vessel and side branch, with the proximal portions of the stents deployed concentrically inside one another. In practice, this results in the proximal portion of the vessel having two layers of stent and the area distal to the bifurcation having just one layer. The technique for performing a culotte technique is perhaps the most demanding of all dedicated approaches, both in terms of wire technique and deployment sequence of the stents. Despite this challenge, the procedure times, fluoroscopic times, and contrast volumes are similar between this dedicated approach and others [20]. To perform a culotte, both the main vessel and side branch are wired and predilation is performed. Next, the side branch stent is deployed so it extends back across the ostium of the side branch into the main vessel. This traps the wire which was in the main branch outside the side branch stent. An additional wire is then used to cross into the lumen side branch stent, exiting the stent via a side cell into the downstream main vessel. At this point the trapped wire can be removed and the main branch stent can be delivered over the newly placed main vessel guidewire. Note that the proximal portion of the main vessel will now have two stents concentrically positioned. Prior to deployment of the main vessel stent, the wire should be removed from the side branch so as not to pin it between two layers of stent. Once the side branch wire is out and the main vessel stent is deployed, the side branch can be recrossed and final kissing balloon angioplasty is then performed (Fig. 22.7). Functionally, the culotte technique has been shown to have less in-stent restenosis when compared with the crush technique, with no difference in overall major adverse cardiac events [20].
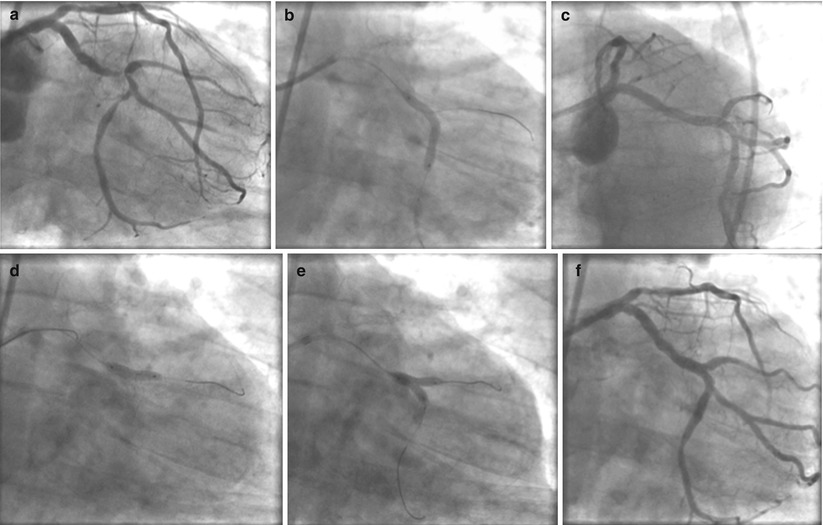

Fig. 22.7
(a–f) Culotte (or “Y”) stenting is a dedicated approach for bifurcation lesions
Randomized Data for Bifurcation Approaches
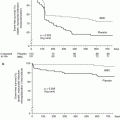
Since the availability of drug-eluting stents, a number of randomized trials have been performed to assess an optimal approach to bifurcation stenosis, primarily comparing the provisional approach to a dedicated two-stent strategy (Table 22.1) [15]. Although there is significant heterogeneity between the trial end points, stenting strategies used, lesion characteristics, and side branch bailout approaches, general wisdom holds that for most bifurcations, there is no disadvantage to a provisional approach in terms of clinical outcomes. Moreover, a provisional approach allows for potentially less resource utilization, less procedure time, and less procedure complexity. However, interventionalists need to remember that randomized data is not a substitute for keen clinical judgment in approaching the more complex bifurcation lesions, many of which were not well represented in the randomized trials and may be better served with a dedicated two-stent approach [15].
Table 22.1
Outcomes of patients with bifurcation lesions randomized to Elective Double Stenting (EDS) Versus Provisional Stenting (PS)
Study | NORDIC [7] | BBK [8] | CACTUS [9] | BBC-ONE [10] | ||||
|---|---|---|---|---|---|---|---|---|
Elective (N = 206) | Provisional (N = 207) | Elective (N = 101) | Provisional (N = 101) | Elective (N = 177) | Provisional (N = 173) | Elective (N = 250) | Provisional (N = 250) | |
Clinical Outcome | 6 months | 1 year | 6 months | 9 months | ||||
Primary end point | Death, MI, TVR, or Stent thrombosis | % DS of the SB | Death, MI, TVR | Death, MI, TVF | ||||
3.4 % | 2.9 % | 27.7 ± 24.8 | 23.0 ± 20.2 | 15.8 % | 15 % | 15.2 %* | 8 %* | |