Ameya Puranik1,*, Sonal Prasad2,3,*, Indraja D. Devi1, and Vikas Prasad4 1 Department of Nuclear Medicine, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai, Maharashtra, India 2 Berlin Experimental Radionuclide Imaging Center, Berlin, Germany 3 Department of Nuclear Medicine, Charité‐Universitaetsmedizin, Berlin, Germany 4 Department of Nuclear Medicine, University Hospital, Ulm, Germany Neuroendocrine tumors (NETs) are a large group of heterogeneous tumors arising from enterochromaffin cells of neural crest origin [1]. Most commonly, they are gastroentero‐pancreatic (GEP) in origin followed by bronchopulmonary [2]. Although they are thought to be rare tumors, recently their incidence has increased exponentially; one of the reasons could be the advancement in method for its detection [3]. The prognosis of these tumors is variable and depends on stage, location, and time of detection. Hence, early diagnosis of NET and prompt management is far more important for better survival rates. Still, there are numerous aspects in the biological behavior of the tumor, which are unexplained due to its inherent heterogeneity. NETs are broadly classified as functional and nonfunctional as many of these tumors have an ability to produce and secrete peptide hormones and biogenic amines, which in turn are responsible for their clinical manifestations [4]. Nonfunctional tumors are difficult to diagnose at an initial stage as they are usually asymptomatic and small. They remain undetected due to their indolent course and are either incidentally diagnosed or detected at an advanced stage when there is extensive disease burden [5]. Hence, accurate imaging procedures and relevant investigations are needed for prompt diagnosis and management. The European Neuroendocrine Tumor Society (ENETS) and the World Health Organization have established grading systems that are based on immunohistochemical staining of Ki‐67 protein, a marker of cellular proliferation. Currently, these grading systems stratify tumors into one of three grades: G1 NET (Ki‐67 ≤ 3%), G2 NET (Ki‐67 = 3–20%), and G3 well differentiated NET or poorly differentiated aggressive neuroendocrine carcinoma (Ki‐67 > 20%) [5]. Functional NETs secrete numerous hormones (most common are chromogranin A, gastrin, 5 HIAA, etc. depending on site of tumor), which are responsible for the clinical syndromes. These hormonal levels in the bloodstream can be measured by various serological tests, which in turn help in screening and diagnosis of NETs. These tests are of little importance in nonfunctional NETs due to their nonsecretory nature. Multimodality imaging remains the mainstay of management in these tumors. Endoscopic ultrasound and contrast enhanced ultrasound Indications Whenever possible, contrast‐enhanced ultrasound should be used for the staging of liver disease. Ultrasound‐guided biopsy is often used for pathological and molecular characterization of a lesion. Computed tomography (CT) and magnetic resonance imaging (MRI) are the most commonly used cross‐sectional imaging methods for assessing locoregional as well as metastatic disease involvement, which helps in treatment planning. This is usually the first investigation performed for the assessment of suspected NETs due to its high spatial and temporal resolution, multiplanar reconstruction, and high speed of acquisition. Detailed anatomical information about the locoregional spread of the disease is obtained for surgical planning [9]. As NETs are hypervascular tumors, they are intensely enhanced on contrast‐enhanced CT scans. Hence, multiphase acquisition with bolus tracking of intravenous iodinated contrast injection reveals typical lesions of NETs, which usually enhance on the early arterial phase [4, 9]. The size of the lesion, calcification, necrosis, and pattern of enhancement help to differentiate between benign and malignant tumors as all these features are often seen in later. Small bowel involvement is delineated with help of negative oral contrast. Commonly used agents are mannitol or plain water. The overall sensitivity for the detection of primary tumor is around 70–80% [9]. The main disadvantages of CT are exposure to ionizing radiation and poor soft tissue resolution. It has low sensitivity in differentiation between reactive and metastatic nodal disease. MRI with a 1.5/3 T magnetic field provides focused evaluation of the target organ with better soft tissue contrast and resolution. It is most useful when other imaging features are equivocal. NETs appear hypointense on T1, hyperintense on T2, and brightly enhanced on post‐contrast sequences. Diffusion‐weighted imaging (DWI) helps to differentiate between well and poorly differentiated lesions as the latter show low apparent diffusion coefficient (ADC) values due to high cellularity [10]. MRI has better sensitivity than conventional contrast‐enhanced CT in the evaluation of liver metastases from NETs. It also helps to differentiate other benign liver lesions from true metastases [4, 9]. The main disadvantages of MRI are its longer acquisition time and high cost. MRI cannot be performed in patients with pacemakers, metallic implants or in patients who are claustrophobic. Functional imaging utilizes various compounds tagged with another radioactive compound, which emits radiation, in turn captured by either a gamma camera or a positron emission tomography (PET) machine. There are two basic classes of functional imaging: These functional imaging methods are relatively new and have improved sensitivity and specificity for the diagnosis of NETs and evaluation of metastatic disease burden [11, 12]. Radiopharmaceuticals are indispensable tools for molecular imaging of NETs. The first somatostatin receptor (SSTR) scintigraphies were carried out in 1989 using 123I‐Tyr3‐octreotide [13]. Since its development 111In‐DTPA‐octreotide has been used extensively for staging, restaging, and therapy stratification. However, as PET is more sensitive and has higher spatial resolution than SPECT, efforts were made to develop gallium‐68 (68Ga), a generator‐produced positron emitter labeled SSTR analogue. Furthermore, PET allows us to quantify the amount of tracer uptake expressed as the standardized uptake value (SUV), which plays a major role in the planning of peptide receptor radionuclide therapy (PRRT). For example, PET imaging with 68Ga‐labeled somatostatin agonists is superior to SSTR scintigraphy [14, 15] using 111In‐DTPA‐octreotide or 99mTc‐HYNIC‐TOC. Moreover, the radiation dose is less than 50% of that of 111In‐DTPA‐octreotide [16]. The 2017 European Neuroendocrine Tumor Society guidelines also recommend imaging of GEP NET with SSTR PET/CT (e.g. 68Ga‐DOTATATE or 68Ga‐DOTATOC) depending on the availability of the tracer in a particular clinical set‐up [17]. Some of the most commonly used PET tracers are 18F‐FDG, 68Ga‐DOTATOC, 68Ga‐DOTATATE, and 64Cu‐DOTATATE. A comparison of the basic properties of the three most commonly used PET radioisotopes, i.e. 18F, 68Ga, and 64Cu is given in Table 17.1. When the positrons annihilate with an electron, two photons are emitted in opposite directions, which are detected by the PET scanner. Combinations of new chelators with different PET isotopes have made SSTR imaging very sensitive and promising. PET isotopes produced by generators have gained more popularity recently because of their easy access, affordable price, and low maintenance, whereas running cyclotrons, specifically in centers with low patient numbers, can be costly and high maintenance. However, one of the major disadvantages of 68Ga is its short half‐life, which limits its potential in using the radionuclide for the purpose of dosimetry. In this scenario, long‐lived isotopes, such as 64Cu with a half‐life of 13 hours or 44Sc with half‐life of 3.97 hours, have an advantage. Somatostatin (sst) receptors are G‐protein‐coupled membrane glycoproteins. Till date five subtypes of human SSTRs have been reported, sst1–sst5. sst2 is the most important because it is homogenously expressed at high density at the surface of NET cells in 80–90% of cases [18]. The first reported somatostatin analog was octreotide, a synthetic octapeptide that has shown higher binding affinities for sst2 and sst5 and, to a lesser extent, sst3 [19]. Even small modifications in synthetic peptides readily changes their binding profile to different receptors. Typically, a radiopharmaceutical comprises a peptide chain, a partial or total electron emitter radionuclide and an appropriate bifunctional chelating group, which is able to form stable covalent complexes with the radionuclide as well with the peptide chain by means of proper molecular spacers. Important factors playing a crucial role in the efficacy of a PET tracer are receptor affinity, radiation type, duration, and positron range of the emissions, in both imaging and PRRT. Minor changes in amino acid sequences, conjugation to a chelator, and the type of isotope may lead to significant changes in the affinity toward different receptors [20, 21]. Use of 1,4,7,10‐tetraazacyclodecane‐1,4,7,10‐tetraacetic acid (DOTA), a universal chelator, has allowed the labeling of many different peptides for PET imaging, making it a standard diagnostic tool for molecular imaging of NET. DOTA forms stable complexes with radiometals like 68Ga, 64Cu, 90Y, and 177Lu [22]. Peptides labeled with 90Y and 177Lu are used for radionuclide therapy because of their beta emitting properties.
17
Correlative Imaging in Neuroendocrine Tumors
Introduction
Diagnosis
Indications for radiological imaging in NETs (focus on GEP NETs)
Imaging Tests
Invasive Tests
Noninvasive Tests
Computerized Tomography Imaging
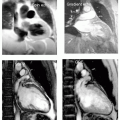
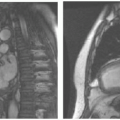
Magnetic Resonance Imaging
Introduction to Functional Imaging
Overview of Radiopharmaceuticals for Imaging and Treatment of NET
Targets for Functional Imaging of NET
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree