Critical Care
The management of critically ill patients becomes more challenging when they are hemodynamically unstable or clinically deteriorating, and the underlying etiology of their condition is unclear. This often occurs shortly after arrival to the ED, but can also occur in other parts of the hospital hours or days after care has been established, with unexpected deterioration of a previously stable patient. In these situations, clinicians are often forced to make treatment decisions before diagnostic tests can be performed. A focused physical examination of the critical organ systems is often inaccurate or misleading. Point-of-care ultrasound can rapidly examine the same organ systems with a higher degree of accuracy.
Point-of-care ultrasound, performed and interpreted by the clinician, was introduced into emergency medicine in the 1980s. In many institutions, it is now the most commonly used diagnostic tool in the initial evaluation of critically ill patients (Figure 8-1). Clinicians using ultrasound can rapidly detect previously occult findings, such as the etiology of cardiac arrest or shock, causes of shortness of breath, sources of sepsis, and volume status and fluid responsiveness. In unstable patients, the ability to obtain this information immediately at the bedside can be lifesaving.
Figure 8-1. Ultrasound machine mounted on an articulating arm in an ED critical care bay. This assures that the machine is always available and ready to use.
This chapter describes how to apply and integrate several different types of ultrasound exams for the diagnosis and management of critically ill patients. The details of how to perform each exam and most of the normal ultrasound findings are detailed in other chapters.
 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS
There are a wide variety of ultrasound applications that have great utility in the evaluation and management of critically ill patients. Many clinicians who manage critically ill patients do not appreciate the extent to which point-of-care ultrasound will improve their diagnostic ability and patient care. In the United States, emergency physicians tend to focus on abdominal, cardiac, and shock applications, while intensivists concentrate on cardiac function and hemodynamic parameters.1 In Europe, clinicians tend to have a much better understanding of pulmonary ultrasound and use it extensively for the benefit of critically ill patients. Those with the most experience often advocate “whole body ultrasonography” in critically ill patients.2
 CLINICAL INDICATIONS
CLINICAL INDICATIONS
Critically ill patients often present with ill-defined disease entities. A variety of ultrasound applications can help clinicians make management decisions when dealing with common problems encountered in the care of critically ill patients.
 Cardiac arrest and near-arrest states
Cardiac arrest and near-arrest states
 Evaluation of undifferentiated hypotension
Evaluation of undifferentiated hypotension
 Assessment of volume status and fluid requirements
Assessment of volume status and fluid requirements
 Assessment of shortness of breath or respiratory distress
Assessment of shortness of breath or respiratory distress
 Evaluation of deep venous thrombosis (DVT)
Evaluation of deep venous thrombosis (DVT)
 Evaluation of abdominal sources of shock or sepsis
Evaluation of abdominal sources of shock or sepsis
 Critical ultrasound-guided procedures
Critical ultrasound-guided procedures
CARDIAC ARREST AND NEAR-ARREST STATES
Critically ill patients are frequently hypotensive and it is occasionally difficult to palpate their pulses (See Chapter 6 Cardiac and Videos 6-1 to 6-5). Managing severe hypovolemia is a precarious situation as the line between hypotension and cardiac arrest is blurred. Assessment of pulses and blood pressure is unreliable in unstable or near-arrest patients. It has been demonstrated that rescuers misjudged pulselessness in 22% of pediatric “cardiac arrest” patients, which led them to withhold cardiac compression in 14% of cases when they were needed and perform compressions in 36% of cases when they were not needed.3 Another study disproved the long-held belief that carotid, femoral, and radial pulses correspond to certain blood pressure measurements, finding instead that there is wide variation and little correlation between pulse palpation and blood pressure.4 Noninvasive blood pressure measurement is equally unreliable in the near-arrest state. A study of 15 resuscitated cardiac arrest patients showed that 33% had unrecordable cuff blood pressures but adequate mean arterial pressures when measured directly.5 In addition, 27% had a cuff blood pressure approaching normal yet a very poor cardiac output. Therefore, without invasive blood pressure monitoring and cardiac ultrasound, clinicians may be flying blind in near-arrest situations, often unsure whether to do chest compressions, give fluid or pressors, or perform other therapeutic interventions. Clinicians who use cardiac ultrasound extensively in these situations often see patients with a normal or hyperdynamic heart but no pulses on physical exam. Also, some patients may show a “normal” automatic blood pressure cuff displayed while cardiac ultrasound shows no activity.
Data support the utility of point-of-care ultrasound in patients with cardiac arrest or near-arrest situations. It is clear that decreased time to diagnosis and therapeutic interventions in these patients results in improved patient outcomes.6,7 The key is to rapidly diagnose and treat reversible conditions. In patients with pulseless electrical activity (PEA), ACLS guidelines suggest searching for the “5 H’s and 5T’s” (Table 8-1).8
 TABLE 8-1. TREATABLE CAUSES OF CARDIAC ARREST FROM THE 2010 AHA ADVANCED CARDIAC LIFE SUPPORT GUIDELINES
TABLE 8-1. TREATABLE CAUSES OF CARDIAC ARREST FROM THE 2010 AHA ADVANCED CARDIAC LIFE SUPPORT GUIDELINES
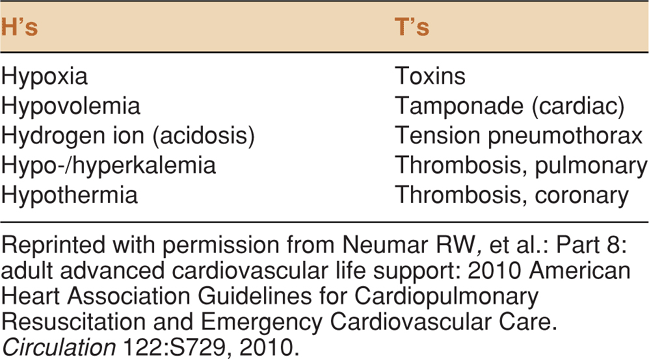
Cardiac function cannot be directly observed without ultrasound. Consequently, potentially treatable causes of cardiac arrest or near-arrest, such as tamponade, pulmonary embolism (PE), severe hypovolemia, and cardiogenic shock, may go undetected. For example, autopsy studies show that PE is often unrecognized clinically when it is the cause of cardiac arrest.9–11 The utility of ultrasound has been demonstrated in identifying reversible causes of cardiac arrest.12–18 One study showed that 12% of cardiac arrest patients had a therapeutic intervention as a direct result of an ultrasound exam.14 Another study showed that 86% of patients with presumed electromechanical dissociation actually had myocardial contractions on cardiac ultrasound.19 The largest report of ultrasound use for cardiac arrest and near-arrest states analyzed 200 patients and demonstrated that ultrasound findings changed management in 78% of cases.13 Also, they found that 35% of patients who were initially thought to be in asystole had cardiac wall motion on ultrasound. Severe bradycardia and subclinical ventricular fibrillation (VF) can both masquerade as asystole. In a study of 18 patients with inhospital cardiac arrest and initial rhythm of asystole, the investigators identified four patients who actually had severe bradyarrhythmia and responded to fluids and inotropic support.18 Also, an echocardiographic assessment for subclinical VF is essential in cardiac arrest. It has long been recognized that the amplitude of VF is dependent on several factors: duration of VF, size and position of electrodes, skin resistance, body habitus, ventricular hypertrophy, and lead gain.20,21 The amplitude of VF may be so low as to appear asystolic on the cardiac monitor. Therefore, it is recommended that the asystole on the cardiac monitor be sonographically confirmed with direct observation of cardiac standstill at the bedside. If fine fibrillations are seen instead, defibrillation should be performed (Table 8-2).
 TABLE 8-2. ULTRASOUND-GUIDED MANAGEMENT OF UNSTABLE PATIENTS: ULTRASOUND ABNORMALITIES AND SPECIFIC TREATMENTS IN CARDIAC ARREST OR NEAR-ARREST SITUATIONS
TABLE 8-2. ULTRASOUND-GUIDED MANAGEMENT OF UNSTABLE PATIENTS: ULTRASOUND ABNORMALITIES AND SPECIFIC TREATMENTS IN CARDIAC ARREST OR NEAR-ARREST SITUATIONS

Withholding chest compressions in pulseless patients is controversial, but investigators of one study modified the advanced life support algorithm based on cardiac ultrasound findings and held compressions briefly in patients who had visible cardiac contractility.15 In patients with PEA who had cardiac activity by ultrasound, they held compressions while rapidly administering 20 IU of vasopressin. Fifteen of sixteen patients (94%) had return of spontaneous circulation and eight (50%) had a good neurologic outcome. This study provided evidence to support the notion that all pulseless patients may not need chest compressions.
EVALUATION OF UNDIFFERENTIATED HYPOTENSION
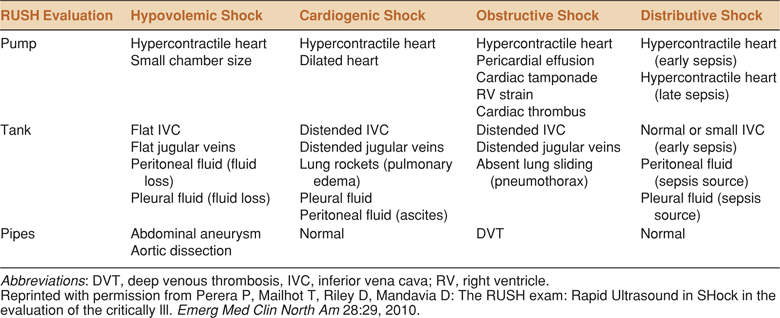
Point-of-care ultrasound is a powerful tool that can help clinicians determine the etiology of shock. Shock is traditionally classified into one of the five categories: hypovolemic, cardiogenic, distributive, obstructive, and neurogenic. Ultrasound provides objective, visual, and recordable evidence to help classify shock. The initial ultrasound exam usually concentrates on the heart, lungs, and inferior vena cava (IVC). Cardiogenic shock is suspected with sonographic findings of a poorly contractile left ventricle, distended IVC, and B-lines on thoracic scan. Obstructive shock is indicated by a distended IVC, lack of B-lines, and a hyperdynamic left ventricle. Right ventricular distention suggesting PE or large pericardial effusion may further elucidate the cause of obstructive shock. Finally, a collapsed IVC coupled with a hyperdynamic poorly filled left ventricle and lack of B-lines indicates hypovolemic or distributive shock (see Table 8-3).
 TABLE 8-3. SUMMARY OF IMPORTANT CARDIAC AND THORACIC ULTRASOUND FINDINGS IN SHOCK
TABLE 8-3. SUMMARY OF IMPORTANT CARDIAC AND THORACIC ULTRASOUND FINDINGS IN SHOCK

In addition, ultrasound examination of the pleural spaces, intraperitoneal space, aorta, deep veins, and the urologic and biliary systems may be useful for identifying hemorrhage, infection, or obstruction. The RUSH (Rapid Ultrasound in SHock) exam is an algorithmic approach to categorization of shock based on a similar concept of “whole body” ultrasound (Table 8-4).23 The benefits of a comprehensive approach to point-of-care ultrasound for shock in order to quickly narrow the differential diagnosis and focus initial management have been demonstrated.6,24
 TABLE 8-4. THE RUSH (RAPID ULTRASOUND IN SHOCK) PROTOCOL. AN ALGORITHM BASED ON THE CONCEPT OF WHOLE BODY ULTRASOUND. RUSH INCLUDES ULTRASOUND OF THE HEART (PUMP), THE INFERIOR VENA CAVA, CHEST AND ABDOMEN (TANK), AND THE AORTA AND DEEP EXTREMITY VEINS (PIPES)
TABLE 8-4. THE RUSH (RAPID ULTRASOUND IN SHOCK) PROTOCOL. AN ALGORITHM BASED ON THE CONCEPT OF WHOLE BODY ULTRASOUND. RUSH INCLUDES ULTRASOUND OF THE HEART (PUMP), THE INFERIOR VENA CAVA, CHEST AND ABDOMEN (TANK), AND THE AORTA AND DEEP EXTREMITY VEINS (PIPES)
In critically ill patients, cardiac ultrasound starts with a visual assessment of the overall cardiac function and relative chamber sizes. Severe abnormalities, such as hypovolemia, tamponade, and massive PE, are usually recognized with visual inspection and do not require specific measurements (Figure 8-2).
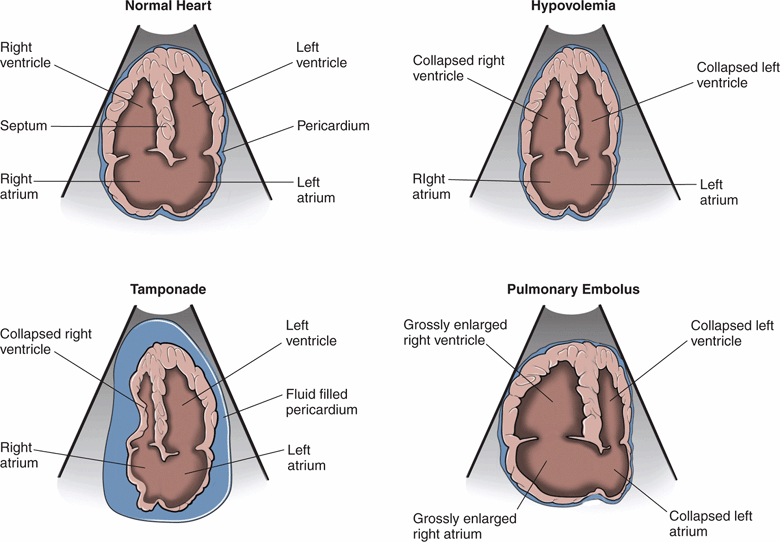
Figure 8-2. Relative cardiac chamber size in different shock states.
There are several ways to measure left ventricular function with echocardiography, but a qualitative visual estimation of global left ventricular function is best in critical situations because it is faster and just as accurate as doing measurements.25–27 Also, it has been clearly shown that emergency physicians and intensivists can accurately estimate left ventricular function using point-of-care ultrasound.28,29 Visual estimation is used to classify global left ventricular contractility into three groups: poor, normal, or hyperdynamic. This determination, though deceptively simple, is profoundly useful in critically ill patients, and with practice, noncardiologists are able to make this estimation with sufficient accuracy.
Poor left ventricular contractility suggests a cardiogenic etiology in patients with shock. Clinicians should remember, however, that all decompensated shock states will eventually result in decreased left ventricular performance, so it is prudent to keep a broad differential and search for other potential sources of shock when indicated. Regardless, it is critical to know whether diminished cardiac function is at least contributory to the shock state. Transthoracic echocardiography has been demonstrated to be 100% sensitive and 95% specific for identifying cardiogenic shock.30
By contrast, a hyperdynamic, vigorously contracting left ventricle suggests a physiologic compensation for hypovolemic, distributive, or obstructive shock. Time constraints may limit quantifiable echocardiographic measurements, but gross estimations of left ventricular contractility, E-point septal separation (EPSS), left ventricular and right ventricular filling, and IVC indices may be quickly obtained (see Chapter 6, “Cardiac,” for cardiac measurements). A small, poorly filled and vigorously beating heart coupled with a narrow, collapsed IVC will usually be evident in patients with severe hypovolemia. In severe cases, complete obliteration of the left ventricular cavity in systole will be seen.
A hyperdynamic left ventricle with increased ejection fraction may develop without tachycardia and may be a warning of fluid loss and pending decompensation. These findings should prompt the physician to rapidly seek etiologies of shock that may be reversible if discovered and treated. This may occur in the setting of trauma, hemorrhage, sepsis, diabetic ketoacidosis, hyperosmolar nonketotic coma, or a host of other clinical scenarios. Clinicians should be mindful of preexisting heart failure, use of beta-blockers or calcium channel blockers, or later stages of shock associated with metabolic decompensation that may prevent a compensatory hyperdynamic state. The left ventricle may also be small and hyperdynamic in shock caused by PE or cardiac tamponade. In hypovolemic and distributive shock, poor left ventricular filling is due to central volume depletion. In obstructive shock, this may be due to a large PE resulting in a right ventricular outflow obstruction preventing left heart filling.31
Cardiac tamponade is a clinical and sonographic diagnosis.32–37 Be aware that physical examination findings of tamponade are inconsistent; Beck’s triad is not usually present; and pulsus paradoxus may be absent in patients with preexisting left ventricular dysfunction, atrial septal defect, regional tamponade, and positive pressure ventilation.33,38 The combination of shock (without another clear source) and a moderate or large pericardial effusion is an indication for emergent pericardiocentesis. Since ultrasound-guided pericardiocentesis is a safe procedure, delaying it while trying to obtain more specific ultrasound findings is generally not prudent. Volume resuscitation may be a temporizing measure but once tamponade is diagnosed the effusion should be removed as soon as possible. Patients with simple fluid are candidates for ultrasound-guided pericardiocentesis; those with penetrating trauma or pericardial clot are usually best served with an emergent thoracotomy.39 Also, it is generally not acceptable to use the landmark (or “blind”) technique for pericardiocentesis if ultrasound guidance is available.40–43
It is common to see sonographic signs of impending tamponade in patients who are normotensive. Sonographically, the first sign of tamponade is collapse of the right atrial free wall during systole (Figure 8-3). As pericardial pressure increases, impaired filling of the right ventricle will also be evident, as well as collapse of the right ventricular free wall during diastole. These changes may be difficult to appreciate in real-time B-mode (2D) imaging but will be more apparent if the video is frozen and rolled backward slowly. A distended IVC that does not collapse with inspiration is also a sensitive sign of current or impending tamponade.44
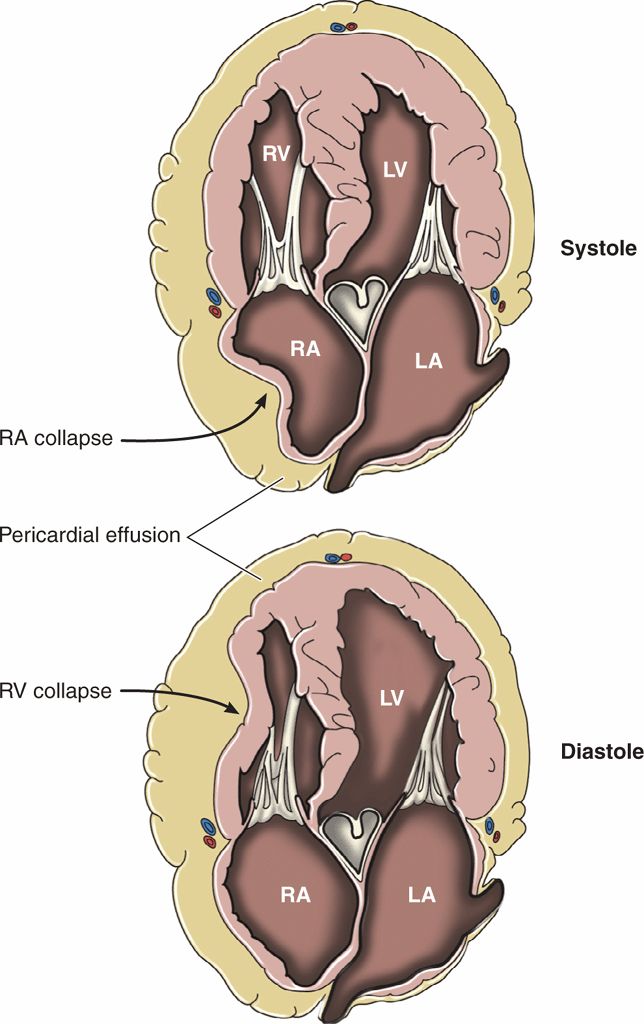
Figure 8-3. Physiology of cardiac tamponade.
PE is often a difficult diagnosis to make clinically, especially if the patient cannot give history of shortness of breath or chest pain. There are no pathognomonic signs on physical exam, and leg swelling is only clinically evident in about 4% of cases. The diagnosis is clinically missed in up to 84% of cases when it is the cause of cardiac arrest.45 Point-of-care ultrasound is invaluable in the early detection of massive PE and obstructive shock. Classic echocardiographic findings of a massive PE are a thin-walled, dilated, and hypokinetic right ventricle, with bowing of the septum into the left ventricle (Figure 8-2). Patients with chronic pulmonary hypertension may also have a large, poorly-functioning right ventricle, but it will have a thick free wall (see Chapter 6, “Cardiac”). Several studies have demonstrated that ultrasound findings of acute right heart strain have only moderate sensitivity (41–66%) but excellent specificity (87–91%) for the diagnosis of massive PE.46–50 Mc-Connell’s sign, described as diffuse hypokinesis of the right ventricular free wall with apical sparing, is a very specific indicator of PE and may help differentiate acute from chronic right heart strain.50 Another sonographic sign of pulmonary artery obstruction is a distended IVC that does not collapse with inspiration.
Patients with sonographic signs of acute right heart strain consistently have a massive clot load and severe pulmonary artery obstruction.51,52 These patients have a high mortality rate, so they may be candidates for surgical embolectomy or thrombolytic therapy.47,53–56 Patients who are in shock (systolic BP <90 mm Hg) and have sonographic signs of massive PE are definitely candidates for thrombolytic therapy.57 Several studies have demonstrated improved patient outcomes with thrombolytics in this scenario.58
ASSESSMENT OF VOLUME STATUS AND FLUID REQUIREMENTS
Physical examination findings and vital signs are notoriously inaccurate for estimating volume status and fluid needs. Sonographic assessment of the size and collapsibility of the IVC is widely used as an indicator of volume status and fluid needs. A small IVC (<1 cm) that completely collapses with inspiration is a reliable indicator of low central venous pressure and a good indicator of hypovolemic or distributive shock (Figure 8-4A). A large IVC (>2 cm) with no inspiratory collapse during forcible inspiration or sniffing is a good indicator of a high central venous pressure and a good predictor of heart failure (Figure 8-4B). This will also be seen with obstructive shock due to a massive PE and in cardiac tamponade. Be aware that patients with chronic pulmonary hypertension will have a large fixed IVC regardless of their hydration status. At the extremes and in intubated or chronically ill patients, IVC measurements are often confusing and they may not be good indicators of fluid requirements. Also, IVC measurements should not be used in isolation but rather in unison with other clinical and ultrasound findings.
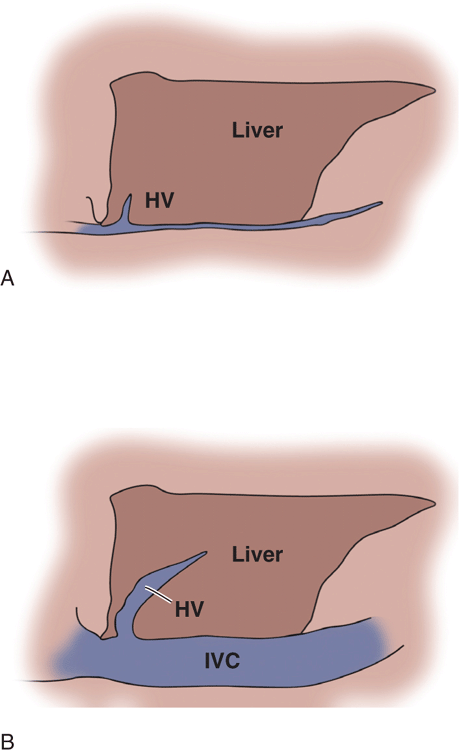
Figure 8-4. Drawing of the IVC at the extremes of volume status. (A) Small collapsing IVC. (B) Distended IVC and hepatic vein with no respiratory collapse.
Sonographic assessment of the heart is critical to determine volume status and the need for fluid resuscitation. Point-of-care cardiac ultrasound with a subjective assessment of hyperdynamic cardiac function and left ventricular end-systolic collapse has been found to be a better indicator of hypovolemia than central venous pressure or IVC size or collapsibility.59 One study demonstrated that visual evaluation of hyperdynamic left ventricular function predicted volume responsiveness with a sensitivity of 71–100%.60 Hyperdynamic left ventricular function is defined as the endocardium of the opposing ventricular walls coming in close proximity or touching during systole (Figure 8-5). Be aware that the opposite finding, a large, poorly-functioning left ventricle, does not necessarily mean that the patient is overloaded with fluid (Figure 8-6). Even patients with severe left ventricular dysfunction sometimes need fluid resuscitation. Patients with chronic left ventricle failure (systolic or diastolic) have chronically elevated left ventricular filling pressure and may deteriorate rapidly if they become hypovolemic, but they are also at risk of volume overload. Fluid management decisions in these patients are difficult, and it is best to measure indices that have been proven to predict fluid responsiveness (see Chapter 6, “Cardiac”). A simple way to estimate fluid status is to do a pulmonary ultrasound exam to help differentiate patients with signs of pulmonary edema from those who may need fluid resuscitation.
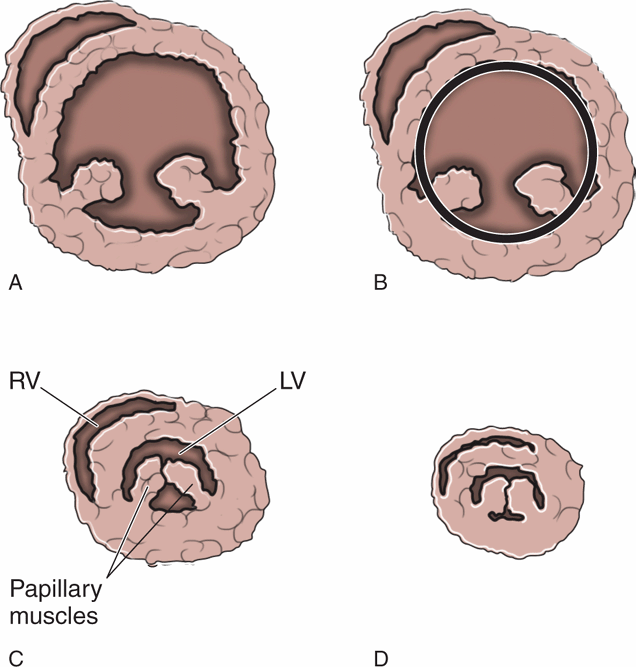
Figure 8-5. Left ventricular area. (A) Short-axis cardiac view at the level of the papillary muscles. (B) Measurement of the left ventricular end-diastolic area (LVEDA). (C) Significantly decreased end-systolic area with “kissing papillary muscles.” (D) Systolic obliteration of cavity of the left ventricle. LVEDA <10 cm2, kissing papillary muscles, and systolic obliteration of the left ventricle are all indicators of significant volume depletion. The parasternal long axis, apical four-chamber, and subcostal views are also helpful for identifying hyperdynamic left ventricular function.
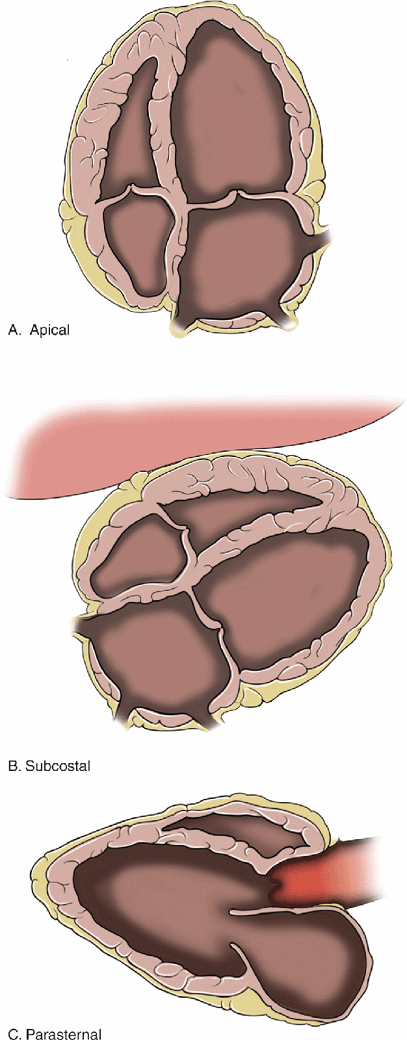
Figure 8-6. Anatomic changes associated with chronic left ventricular dysfunction A: 4-chamber view, B: Subcostal view, C: Parasternal long axis. The left ventricle and left atrium are significantly dilated. These are very common findings in patients with chronic heart disease. Patients with these findings have chronically elevated filling pressures and it is often difficult to determine their fluid requirements.
Pulmonary ultrasound is one of the best ways to determine volume status, especially for recognizing fluid overload (Figure 8-7). The finding of diffuse and bilateral B-lines is extremely sensitive for the diagnosis of pulmonary edema and extravascular lung water (which is associated with increased pulmonary wedge pressure and left ventricular systolic and diastolic dysfunction).61–65 One study found a direct linear correlation between an increased number of B-lines and increased intravascular volume as measured by invasive techniques (pulmonary capillary wedge pressure and extravascular lung water by pulse contour cardiac output).66 Another study found that the number of B-lines decreased in real time as intravascular fluid volume decreased during hemodialysis.67 Lung ultrasound is more sensitive for detecting early pulmonary edema than chest radiography or the clinical symptom of dyspnea.63,66,67
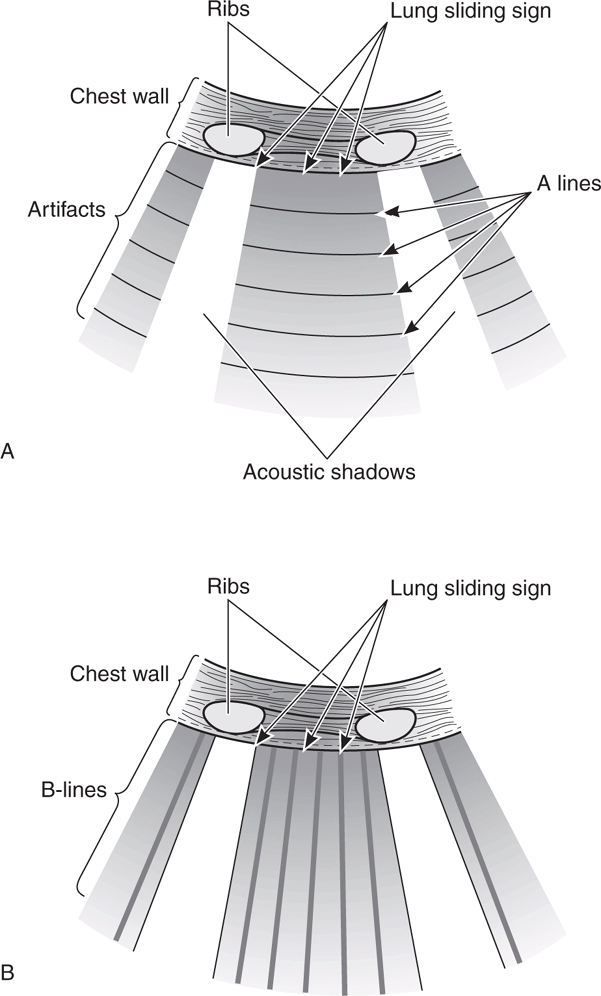
Figure 8-7. Pulmonary ultrasound to evaluate for extravascular lung water. (A) Normal “dry lung” has a prominent A-line pattern. (B) Prominent B-line pattern is consistent with “wet lung” (alveolar-interstitial syndrome: pulmonary edema or ARDS).
Prominent B-lines can also be seen in ARDS, but the distribution will be patchy rather than diffuse (see Chapter 7, “Pulmonary”).
Although a rapid sonographic assessment of the IVC, heart, and lungs provides a good initial estimate of volume status, this information does not necessarily predict fluid responsiveness. When determining fluid requirements, it is important to understand the concept of fluid responsiveness and how it differs from central venous pressure or filling pressures. Historically, central venous or filling pressures were measured and used to make decisions about fluid resuscitation. However, significant evidence shows that these parameters are poor predictors of fluid responsiveness.68,69 Fluid responsiveness is defined as an increase in cardiac output with a fluid challenge. Measuring parameters that predict fluid responsiveness is ultimately the best way to make decisions about the need for fluid resuscitation (see section “Volume Status and Fluid Responsiveness” in Chapter 6, “Cardiac”).
ASSESSMENT OF SHORTNESS OF BREATH OR RESPIRATORY DISTRESS
In patients with respiratory distress of unknown etiology, it is important to initiate treatment as quickly as possible. Chest radiography is the traditional initial imaging choice, but awaiting results would often cause an unacceptable delay in initiation of treatment. One study showed high concordance between chest ultrasound and chest radiography in patients presenting with acute dyspnea.70 In addition, ultrasound results were available much more quickly than radiography results. The authors suggested that chest ultrasound could replace the standard chest radiograph in this setting (See Videos 7-1 and 7-2).
Pneumothorax
Missed pneumothorax is a common cause of morbidity and mortality.71 For patients who are rapidly deteriorating, there is good evidence that ultrasound is better than chest radiography for diagnosing pneumothorax. One study found that the sensitivity of ultrasound for detecting pneumothoraces was 98% compared with 75% for radiography.72 Several studies have shown that the negative predictive value of ultrasound for ruling out a pneumothorax is 100%.72–78 Most importantly, ultrasound is much quicker than chest radiography. One study showed that chest ultrasound was performed in an average of 2 minutes, while chest radiography took 20 minutes.78 A single sagittal window through the upper lung fields is dependable, but sensitivity is increased by scanning several areas along the anterior and lateral chest. In the presence of a pneumothorax, the clinician will appreciate an absence of lung sliding and comet-tail artifact at the pleural interface. This may be accentuated by obtaining an M-mode tracing at this level. It is important to remember that several other clinical scenarios may cause an absence of lung sliding, including pulmonary blebs, adhesions, and atelectasis.79,80 In addition, the same concept that is used to rule out or diagnose a pneumothorax, the presence or absence of the lung sliding sign can be used to diagnose or rule out a main stem or esophageal intubation.81,82
Detection of Main Stem Intubation
When critically ill patients are intubated, it is important to assess the position of the endotracheal tube. Auscultation of breath sounds is inaccurate for detecting both esophageal intubation and main stem intubation.83–85 Therefore, waveform capnography is the gold standard for detecting esophageal intubation, and chest radiography is the gold standard for determining endotracheal tube depth and diagnosing main stem intubation. However, the use of chest radiography usually causes a delay in the diagnosis of main stem intubation. This delay may be avoided by looking for the lung sliding sign immediately before and after intubation. The presence of bilateral lung sliding after intubation is an accurate indicator of good endotracheal tube position.81,82,86,87 Unilateral lack of lung sliding may indicate a main stem intubation and the endotracheal tube depth should be checked.81,87 If lack of sliding is thought to be the result of a main stem intubation, the endotracheal tube can be pulled back as the operator watches for the return lung sliding in real time.
Pulmonary Edema
Pulmonary edema is a common problem in critically ill patients. However, “classic” diagnostic characteristics such as rales, jugular venous distention, and interstitial edema on chest radiograph are absent in more than 50% of patients who present with dyspnea from acute congestive heart failure.88,89 Pulmonary edema is very straightforward to recognize on chest ultrasound. It causes thickened subpleural intralobular septa that result in long vertical hyperechoic lines (B-lines) emanating from the pleural interface (Figure 8-7B).90 B-lines are very sensitive for pulmonary edema.63,67,91 Some B-lines are present at the lung bases in 27% of patients without pulmonary edema, but are a very sensitive indicator of pulmonary edema when prominent in the upper lung fields.64
Multiple B-lines visualized in the upper lung fields are very specific for pulmonary edema, and very accurate for discriminating pulmonary edema from chronic obstructive pulmonary disease (COPD) in patients with acute dyspnea and severe respiratory distress.63,92 B-lines precede the development of radiographic findings of pulmonary edema and correlate well with pro-BNP levels.91 Also, they resolve as fluid is removed from the body during hemodialysis.67 In the hypotensive patient, B-lines are 94% sensitive and 84% specific for a cardiac etiology.93
Pleural Effusions
Ultrasound is extremely sensitive (96.2%) and specific (100%) for the diagnosis of fluid in the hemithoraces, detecting as little as 20 mL.94,95 Pleural effusions may be present in a wide variety of pathologies including trauma, congestive heart failure, malignancy, renal failure, and pneumonia. In the setting of chest trauma and hypotension, a pleural effusion may signal hypovolemic shock due to a hemothorax. In a patient with poor cardiac function and B-lines, pleural effusions are consistent with a diagnosis of heart failure. A pleural effusion in a patient with pleuritic chest pain may increase suspicion for a PE. The clinician should interpret the finding of pleural effusion in the context of other clinical and sonographic information.
Pericardial Effusions
It is well known that patients with a pericardial effusion often present with nonspecific symptoms or with a primary complaint of dyspnea.37,38 One study evaluated the use of point-of-care echocardiography on 103 ED patients with unexplained dyspnea and found that 14 had pericardial effusions. Four patients had large effusions requiring pericardiocentesis and three had moderatesized effusions that were treated conservatively. It was recommended that ED patients with unexplained dyspnea be evaluated for a pericardial effusion.96
EVALUATION OF DEEP VENOUS THROMBOSIS
Ultrasound of the peripheral venous system has become the modality of choice for the diagnosis of DVT (See Video 17-1). Multiple studies have shown that clinicians can accurately detect DVTs using a modified compression technique, with a sensitivity ranging from 98% to 100% compared with a comprehensive duplex exam performed in a vascular lab (Figure 8-8).96–98 In the management of critically ill patients, this exam is most useful when an acute PE is suspected.99 In one study of 383 patients with known PE who underwent compression ultrasound, a lower extremity DVT was found in 289 (76%).100 Signs or symptoms of DVT were present in only 31%.
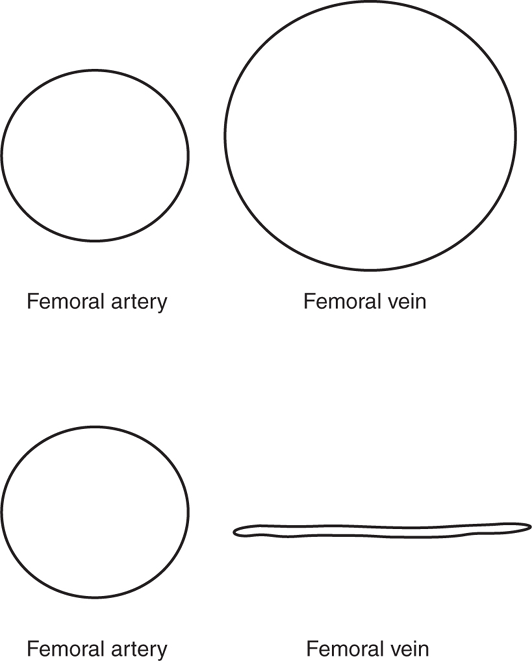
Figure 8-8. Simple compression test for DVT. The vast majority of DVTs are located in either the proximal femoral vein or at the popliteal vein. Locating and compressing the veins in these locations is an accurate way to rapidly evaluate for DVT. This is an example of normal complete compression of the femoral vein, which rules out DVT at this location.
Point-of-care ultrasound may be useful in the ICU setting when surveying for the development of hospital-acquired DVTs because the true prevalence of acute DVTs in the ICU setting is unknown and may be as high as 60% depending on the patient population, detection methods, and the application of surveillance programs.101–103 Upper extremity sonography is important for patients with prolonged internal jugular, subclavian, basilic, or brachial catheters.
EVALUATION OF ABDOMINAL SOURCES OF SHOCK OR SEPSIS
Abdominal ultrasound is often overlooked by critical care practitioners, but it is very useful for identifying several specific life-threatening causes of shock and sepsis.
Ruptured Abdominal Aortic Aneurysm
Patients with ruptured abdominal aortic aneurysm (AAA) are initially misdiagnosed in 30–60% of cases.104,105 This is an avoidable problem, because point-of-care ultrasound is nearly 100% accurate for the diagnosis of aortic aneurysm (See Video 9-1).106,107 In addition, ruptured AAA is a relatively common cause of cardiac arrest.108 Although some physicians argue that cardiac arrest resulting from ruptured AAA presents a miniscule chance of survival and operative intervention is a waste of resources, this is not well supported in the literature. One study demonstrated 28% (11 of 39) survival in patients with ruptured AAA presenting as cardiac arrest.108
Intra-Abdominal Free Fluid
The utility of the FAST exam is well described and accepted in the trauma literature, primarily because of the ease of detecting intraperitoneal fluid. It is highly sensitive for intra-abdominal hemorrhage in hypotensive trauma patients (See Videos 5-1 to 5-6).109 Several studies report the ability of the FAST exam to detect intraperitoneal fluid collections from 250 to 620 mL, with overall sensitivity of 79% and specificity of 99%.109–112 It is a small cognitive leap for most physicians to use the same exam for other nontraumatic abdominal catastrophes that present with intra-abdominal fluid collections. In unstable pregnant women, a positive FAST exam coupled with lack of intrauterine pregnancy is extremely sensitive for ectopic pregnancy and mandates immediate operative intervention. Abdominal free fluid is estimated to be present in 26% of patients with intestinal ischemia due to superior mesenteric artery occlusion.113
Cholecystitis
In elderly patients with sepsis and hypotension but without a clear infectious source, sonography of the gallbladder is recommended (See Videos 10-1 and 10-2). In cases of abdominal sepsis in the elderly, approximately 25% are due to acute cholecystitis and cholangitis.114 Diagnosis is delayed in as much as 33% of these patients due to lack of physical exam findings.115,116 A point-of-care ultrasound of the gallbladder may elucidate the etiology of undifferentiated septic shock, especially in the elderly patient unable to provide a history. Acute acalculous cholecystitis, although infrequent as an overall cause of cholecystitis, is seen more frequently in the ICU setting postoperatively or as a consequence of sepsis, trauma, or major burns. The sonographic evaluation of acalculous cholecystitis involves the same findings as calculous cholecystitis: a thickened gallbladder wall with pericholecystic fluid. An enlarged gallbladder, exceeding 9 cm longitudinally and 5 cm transversely is another typical feature. Although gallstones are absent, sludge should be present in the lumen of the gallbladder. Murphy’s sign may be unreliable or absent, likely due to ICU sedation and pain control.
One study evaluated the routine use of gallbladder ultrasound in 53 intubated ICU patients and found three septic patients with acalculous cholecystitis in whom surgical intervention was performed and judged to be lifesaving.117
Hollow Viscus Perforation
An intraperitoneal air collection may be so large that underlying structures are obscured by dirty shadows. Smaller amounts of fluid may be detectable as bright reverberation artifact arising from the inner wall of the peritoneum. These reverberation artifacts, caused by air adjacent to the peritoneal wall, will be displaced with gentle pressure.118 These findings are usually best detected in the right upper quadrant between the anterior abdominal wall and the liver where there is no intervening bowel.119 Using these methods, ultrasound has been demonstrated to be 86% sensitive and 99% specific for detecting pneumoperitoneum. In contrast, abdominal plain films are reported to be 50–60% sensitive for intraperitoneal free air.120–123
Urosepsis
Complications of pyelonephritis, including abscess formation and emphysematous pyelonephritis, are commonly missed causes of sepsis, especially in the elderly population. Only 15–25% of perinephric or intrarenal abscesses were diagnosed at the time of admission.124–126 The clinician should be rigorous in the bedside renal ultrasound evaluation of patients with sepsis with presumed pyelonephritis as a source (See Videos 12-1 and 12-2). Patients with diabetes, renal stones, immunosuppression, and renal failure are especially at risk.
Source control is an important part of early goal-directed therapy, and is known to decrease mortality in patients with sepsis.127 The early identification of abscess or emphysematous pyelonephritis can expedite surgical intervention, either by percutaneous drainage or open nephrectomy. Ultrasound has been shown to be an effective initial tool for the detection of retroperitoneal abscess.128 A renal abscess is typically a solitary, round hypoechoic mass with posterior acoustic enhancement, typically containing internal septations or mobile debris. Emphysematous pyelonephritis is a rare but deadly infection, occurring more commonly in diabetic or immunocompromised patients. Gas formation by bacteria in the kidneys causes dirty shadowing on ultrasound. This finding should prompt CT scanning; early emergent surgical consultation and intervention may be lifesaving for these patients.
Acute Renal Failure
Etiologies of acute renal failure are often divided into pre-renal, intrinsic, and post-renal causes. Most causes of intrinsic renal failure do not cause any sonographic abnormality, though occasionally the clinician may detect the absence of a kidney, or small, atrophic, hyperechoic kidneys. These suggest a more chronic nature of the renal failure.
Ultrasound is extremely useful in the diagnosis of postobstructive renal failure. Although only accounting for 5% of all causes of renal failure, obstructive renal failure is the most imminently reversible and most amenable to ultrasound investigation. In the presence of bilateral hydronephrosis, the clinician should assess for bladder outlet obstruction. This will usually include an ultrasound exam of the bladder for masses, clot (possibly around a urinary catheter), or prostatic hypertrophy. Bilateral hydronephrosis in patients with acute renal failure requires emergency decompression of the kidneys.
CRITICAL ULTRASOUND-GUIDED PROCEDURES
Ultrasound guidance of procedures allows clinicians to improve their success with procedures that are not routinely performed, such as pericardiocentesis and transvenous pacemaker placement. In addition, ultrasound guidance allows increased speed and accuracy, and decreased complications, with procedures that are routinely performed such as placement of a central line, paracentesis, and thoracocentesis (see Chapter 21, “Vascular Access,” and Chapter 22, “Additional Ultrasound-Guided Procedures”).129
Troubleshooting and Ensuring Proper Placement of Central Venous Catheters
Ultrasound guidance has replaced the landmark technique as the standard of care in placing central venous catheters, with a reduction in failed attempts and complications (see Chapter 21, “Vascular Access”).129 However, the risk of carotid artery puncture or cannulation is present even when proper technique is followed.130 Also, malposition of a catheter into an aberrant location, outside of the central circulation, occurs in 2–50% of procedures depending on the site of venous puncture.131 In addition to ultrasound guidance of the needle puncture and direct observation of the catheter inside the vein at the puncture site, there are two other ultrasound techniques that can be used to ensure that the tip of the catheter is in the proper venous location.
Cardiac Bubble Test
Performing a cardiac bubble test is an effective method to assure venous catheter placement and rule out arterial catheter placement.132 This test involves observing the heart while the catheter is flushed with 10 mL of normal saline. Any cardiac view that shows the right atrium or right ventricle is sufficient.
Visualization of the Guidewire in the IVC
Another method to assure proper central venous catheter placement is to visualize the guidewire inside of the IVC during the procedure. Visualization of the guidewire within the IVC proves that the wire is in a vein (not an artery) and also assures that the catheter will end up in the central venous circulation as it is placed over the guidewire. This technique can confirm venous placement of the wire before the dilator is inserted, so it assures that an arterial puncture will not be dilated and cannulated. Some providers argue that overinsertion of the guidewire is dangerous and will cause cardiac sustained arrhythmias, but this practice has been studied and found to be safe.133 Guidewires are straightforward to visualize because their irregular surface reflects ultrasound and any view of the IVC will suffice, although a longitudinal view is usually more visually pleasing.
Ultrasound-Guided Pericardiocentesis
Ultrasound guidance for pericardiocentesis is the preferred technique, as it is safer and more effective than the traditional technique.42 The traditional technique is essentially “blind” and has a high rate of procedural failure and major complications.40 Ultrasound-guided pericardiocentesis has been reported to have a success rate of 97% and a major complication rate of 1.2%.41 The location of needle placement through the chest wall should allow for the shortest distance between the skin surface and cardiac effusion.41–43 Aim the needle toward the largest, most accessible part of the effusion, with the needle trajectory such that the risk of cardiac puncture is minimized.
Ultrasound-Guided Placement of Transvenous Pacer
Ultrasound-guided placement of a transvenous pacing catheter enables more accurate placement than the traditional approach of observing electrical waveforms. When compared against fluoroscopy, sonographic guidance allows shorter time to pacing with a lower incidence of complications and a lower incidence of pacemaker malfunction during pacing.134
Confirmation of IV position of a central venous catheter may be achieved with the rapid instillation of 10 mL of agitated normal saline while observing the heart for bubbles moving through the right ventricle.132 Confirmation of both IV position and proper placement toward the central circulation can be achieved by visualization of the guidewire in the IVC during the procedure.
Ultrasound-Guided Cricothyroidotomy or Tracheostomy
Emergency cricothyroidotomy is a relatively rare procedure. Recent studies report cricothyroidotomy rates of only 0.4–1.2%.121,122 Complication rates are high, ranging from 9% to 40%, with the most frequent being misplacement of the tracheal tube through the thyrohyoid membrane.135,136 Percutaneous tracheostomy is commonly performed in the critical care setting; early complications occur in about 11% of patients.137 Late complications, including tracheal stenosis from inappropriately high placement and erosion into mediastinal vessels from inappropriately low placement, occur in approximately 17% of patients.138
Both cricothyroidotomy and percutaneous tracheostomy techniques rely on palpation of the neck to identify anatomic landmarks. However, anterior neck anatomy is often difficult to palpate. Sonographic assessment of relevant anatomy and guidance of percutaneous needle puncture has been described.139–142 The thyroid and cricoid cartilage, the cricothyroid membrane, and tracheal rings are identified on a sagittal ultrasound window. Point of needle entry may be quickly marked for static ultrasound guidance before the procedure. The mean time to visualization of the cricothyroid membrane has been reported to be 24 seconds and did not vary significantly with patient body habitus.141 Ultrasound guidance increases success of tracheostomy placement.140,142
 GETTING STARTED
GETTING STARTED
Ideally, all clinicians who care for critically ill patients should have significant experience with point-of-care cardiac, pulmonary, abdominal, and vascular ultrasonography. Clinicians who are relative ultrasound novices should concentrate on a handful of applications to get started. The pulmonary exam to assess for pneumothorax, basic cardiac exam to assess for gross function and a pericardial effusion, and ultrasound-guided peripheral and central venous access are ideal starting points.
When using ultrasound in critical care situations, it is important not to interfere with other more important diagnostic tests or patient management. Like the focused physical exam, point-of-care ultrasound should be brief and performed simultaneously with other diagnostic tests and ongoing care.
 COMMON ABNORMAL ULTRASOUND FINDINGS IN CRITICALLY ILL PATIENTS
COMMON ABNORMAL ULTRASOUND FINDINGS IN CRITICALLY ILL PATIENTS
This section demonstrates actual findings from the use of point-of-care ultrasound in critically ill patients. The reader is encouraged to review other chapters within this book for more specific information on interpretation of abnormal ultrasound findings.
CARDIAC ARREST AND NEAR-ARREST STATES
Cardiac imaging may be challenging in critically ill patients. Adequate windows often require the patient to be in the left-lateral decubitus position with the left arm elevated, but this is difficult to accomplish in such scenarios. In these cases, the clinician may choose other windows or settle for suboptimal or nonstandard images. Patients with long-standing obstructive pulmonary disease often have difficult cardiac visualization due to hyperinflation of the lungs and only the subcostal window allows reasonable cardiac images. Sonography during cardiac arrest is difficult because it requires the ability to obtain and interpret technically adequate images quickly; this is possible and extremely useful in clinical practice.143,144 In this situation, cardiac ultrasound should be done quickly and concurrently with the 5-second pause for pulse and rhythm check. The subcostal four-chamber view is usually the “go to” view in cardiac arrest, and the parasternal long-axis position is an excellent alternative when the subcostal view is inadequate.
The objective is to rapidly identify any potentially treatable causes for cardiac arrest and/or profound shock (see Tables 8-1 and 8-2). Common findings are outlined in the following case examples (Figures 8-9 through 8-13).
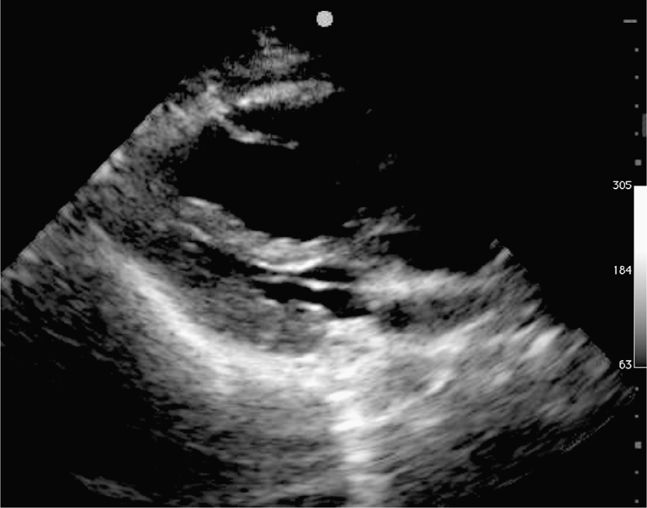
Figure 8-9. Cardiac arrest from massive PE, parasternal long-axis view. The right ventricle (top) is massively dilated and compressing the left ventricle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS CLINICAL INDICATIONS
CLINICAL INDICATIONS GETTING STARTED
GETTING STARTED COMMON ABNORMAL ULTRASOUND FINDINGS IN CRITICALLY ILL PATIENTS
COMMON ABNORMAL ULTRASOUND FINDINGS IN CRITICALLY ILL PATIENTS PITFALLS
PITFALLS CASE STUDIES
CASE STUDIES