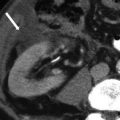
Fig. 27.1
Development of the female genital tract from the paramesonephric ducts and urogenital sinus. (a) Embryologic development of uterus and vagina: both mullerian ducts (pink) fuse in the midline to form uterus. Proximal part of the mullerian duct forms the respective fallopian tubes. Wolffian ducts (green) regress; its distal remnant forming the Gartner duct. (b) Uterovaginal canal (brown) reaches the urogenital sinus (pink area) (1). The vaginal plate develops (2), proliferates (3), and undergoes canalization (4). The vagina is formed by both the mullerian ducts and the urogenital sinus (5)
Ultrasound
Ultrasound is the standard and first imaging modality used to evaluate pelvic structures. Transabdominal ultrasound provides a global survey of the pelvic structures, whereas transvaginal ultrasound provides a high-resolution technique to accurately assess and characterize pelvic lesions. Occasionally, transrectal or transperineal ultrasound may be performed to evaluate pelvic lesions when transvaginal ultrasound is contraindicated.
Sonohysterography
Sonohysterography is mainly indicated in menstruating and postmenopausal women with vaginal bleeding, to differentiate between diffuse and focal causes of endometrial thickening and to further characterize focal endometrial lesions. Sonohysterography should be performed in the follicular phase, at day 10 of the cycle, as the endometrium is very thin at this point of the cycle. If performed later, focal endometrial contour irregularities can mimic small polyps or focal areas of endometrial hyperplasia. No anesthesia is required for the procedure. Painkillers may be prescribed 30 min before the examination. Prior to insertion, a 5- or 7-French hysterosonogram catheter must be flushed with saline to rid it of air, which can cause an echogenic artifact. Under due aseptic precautions, the catheter is then inserted through the cervical os into the endometrial canal, and the balloon tip is inflated with 1–2 ml of saline to hold it in place. The speculum is then removed carefully to avoid dislodging the catheter. The vaginal probe is then reinserted and a 20 ml syringe filled with sterile saline is attached to the catheter. Fluid is instilled while the transducer is moved from side to side (cornua to cornua) in a long-axis projection. The uterus is completely surveyed by ultrasound. Adequate visualization of endocervical canal often requires synchronous delicate progressive collapse of the balloon, and fluid instillation into the canal while the catheter is slowly pulled back
Catheter insertion into the endometrial canal may be difficult. Changing the toe of speculum may change the angle of the cervix and often allow successful catheterization. Cervical stenosis may prevent catheterization, which could be circumvented by the use of a cervical dilator or by threading a catheter without a balloon tip over the guide wire into the canal. Before catheter pullout, synchronous delicate progressive collapse of balloon with slow fluid instillation into the canal should be done while the catheter is slowly pulled back.
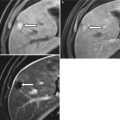
Common technical pitfalls include the following: introduction of air into the catheter, which produces echogenic artifact and may obscure abnormalities; back flow of the injected saline with non-distension of the uterine cavity; and balloon hyperinflation. Flushing the catheter with saline prior to the procedure may avoid air within the catheter. Backflow can be prevented by gently retracting the inflated balloon to occlude the internal cervical os (Fig. 27.2). Overinflation of the balloon can be avoided by appropriately inflating the balloon to the appropriate level (Fig. 27.3). Complications of sonohysterography are minor and include failure to complete the procedure, pelvic pain, vagal symptoms, nausea, and post-procedure fever.
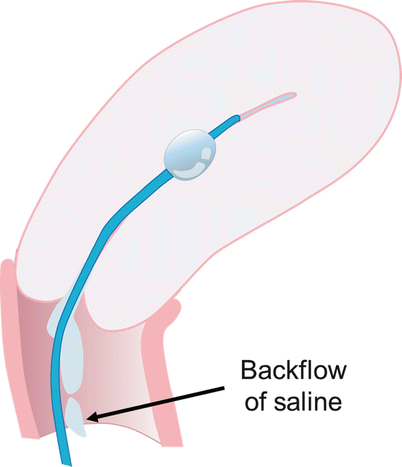
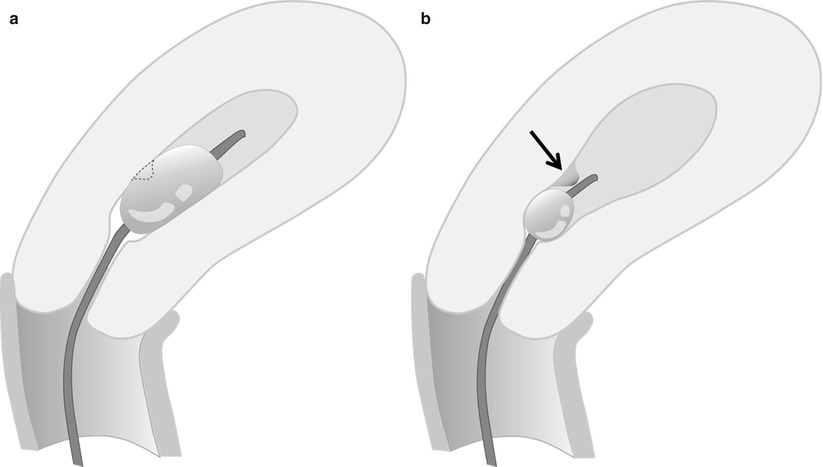

Fig. 27.2
If catheter is too advanced into the uterine cavity and does not occlude the internal os, backflow of saline occurs with non-distension of the cavity

Fig. 27.3
(a, b) Balloon hyperinflation may obscure an underlying pathology. This endometrial polyp (arrow) was initially obscured by balloon hyperinflation, which was revealed by deflating the hyperinflated balloon to appropriate inflation level
Cross-Sectional Anatomy and Imaging Technique
On unenhanced computed tomography (CT) images, all pelvic soft tissue structures have similar density with complete lack of contrast between individual organs. When imaging the uterus, intravenous contrast is necessary and preferably multiphase imaging must be performed. On contrast-enhanced CT scans, differential enhancement may occasionally be identified, with early and homogeneous enhancement of the uterine corpus and vagina compared with delayed enhancement of the cervix; however, due to the presence of enlarged paracervical venous plexuses, it is extremely difficult to analyze the cervix on CT. The ability of magnetic resonance (MR) imaging to provide unique and superior contrast resolution, along with its multiplanar capability, results in excellent anatomical delineation of pelvic structures. In view of this, MR is the preferred cross-sectional modality to evaluate the normal anatomy, embryological variants, and pathologies of the uterine corpus and cervix.
MR Imaging Technique
MR is the current gold standard for noninvasive assessment of uterine corpus and cervix pathologies and may be performed on either 1.5-T or 3.0-T clinical scanners using a phased array pelvic surface coil. For optimal results, patients either are instructed to fast for 4–6 h prior to the examination or are administered an antiperistaltic agent if not contraindicated (hyoscine butylbromide or glucagon) to limit artifacts secondary to small-bowel peristalsis. The patients are also instructed to empty the bladder to reduce artifacts from a distended bladder.
A standard pelvic MR protocol for evaluating the endometrial lining consists of the following: A planning large FOV (250 mm) coronal T2-weighted FSE sequence is performed to identify the orientation of the endometrial lining. Once the endometrial lining is identified, high-resolution T2-weighted fast spin echo (FSE) sequences are obtained at a smaller FOV of 180–200 mm in the sagittal, oblique axial (perpendicular to the endometrial lining) and coronal (parallel to the endometrial lining) planes for evaluation of the endometrial canal (Fig. 27.4). Pre-contrast T1-w axial images with extended field of view are performed to cover the entire pelvis for nodal and bone marrow evaluation. Dynamic gadolinium-enhanced 3D T1-wieghted sequences are obtained in the sagittal or oblique axial (perpendicular to the endometrial lining) planes. The dynamic gadolinium-enhanced 3D T1-weighted sequence is obtained at 1–1.5 mm slice thickness and is used to identify the subendometrial band of enhancement. Diffusion-weighted sequence is obtained in a straight axial plane. Post-contrast T1-weighted fat-suppressed sequences are obtained in the sagittal and oblique axial and coronal planes.
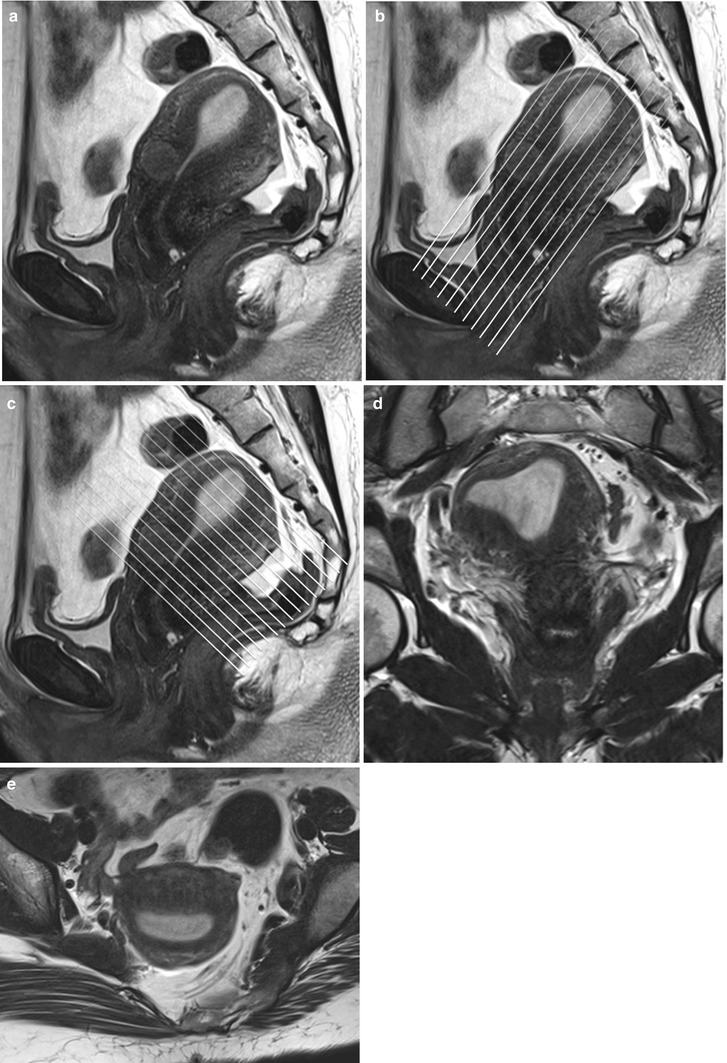

Fig. 27.4
Panel (a) shows a mid-section high resolution T2-w sagittal sequence. Once the endometrial lining is delineated, high resolution T2-w true coronal (b) and axial (c) sequences are planned parallel and perpendicular to the endometrial lining. Panel (d) and (e) are representative true coronal and axial images obtained at the mid-section through the uterine body
A standard pelvic MR protocol for evaluating the endocervical lining consists of the following: A planning large FOV (250 mm) coronal T2-weighted FSE sequence is performed to identify the orientation of the endometrial lining. Once the endocervical lining is identified, high-resolution T2-weighted fast spin echo (FSE) sequences are obtained at a smaller FOV of 180–200 mm in the oblique axial (perpendicular to the endocervical lining) and coronal (parallel to the endometrial lining) planes for evaluation of the endocervical canal (Fig. 27.5). Pre-contrast T1-weighted axial images with extended field of view is performed to cover the entire pelvis for nodal and bone marrow evaluation. Dynamic gadolinium-enhanced 3D T1-weighted sequences are obtained in the sagittal or oblique axial (perpendicular to the endocervical lining) planes. The dynamic gadolinium-enhanced 3D T1-weighted sequence is obtained at 1–1.5 mm slice thickness. Diffusion-weighted sequence is obtained in a straight axial plane. Post-contrast T1-weighted fat-suppressed sequences are obtained in the sagittal and oblique axial and coronal planes. Delayed T1-weighted fat-suppressed images are useful to identify the tumor–myometrial interface, cervical involvement, and nodal lesions.
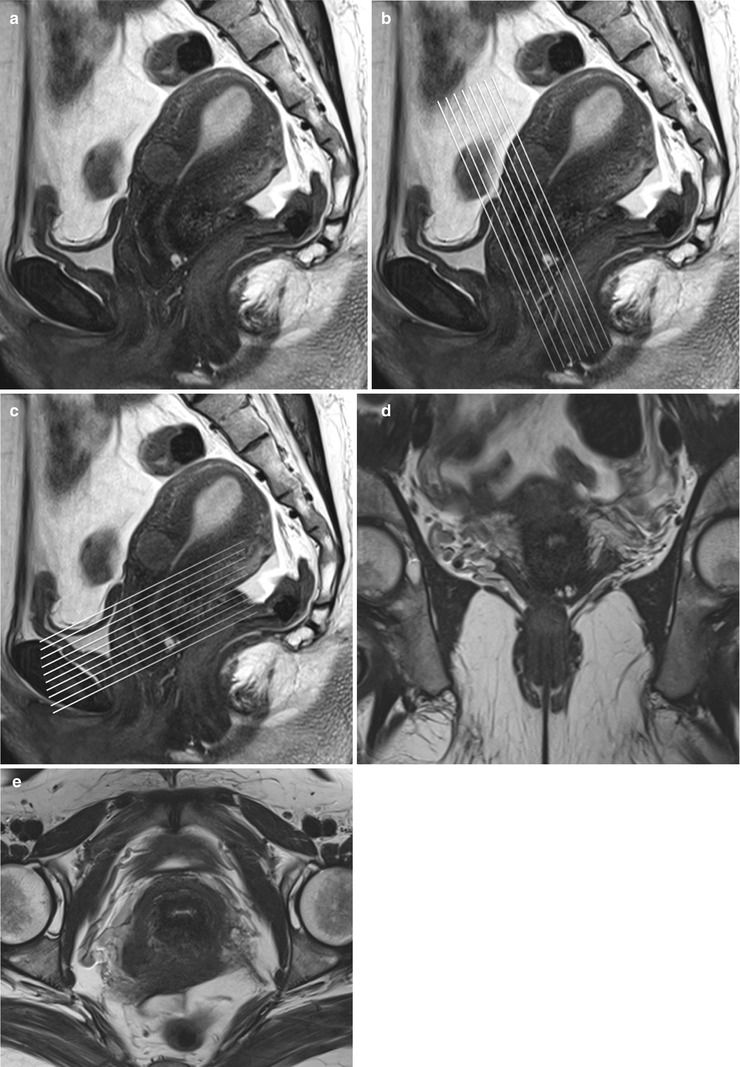

Fig. 27.5
Panel (a) shows a mid-section high resolution T2-w sagittal sequence. Once the endocervical lining is delineated, high resolution T2-w true coronal (b) and axial (c) sequences are planned parallel and perpendicular to the endocervical lining. Panel (d) and (e) are representative true coronal and axial images obtained at the mid-section through the cervix
MR Anatomy of the Uterine Corpus and Cervix
The normal anatomy of the uterine corpus and cervix is best depicted on T2-weighted sagittal images. The endometrial cavity appears as a slit-like T2 hyperintense cleft, surrounded by high T2 signal endometrium. The endometrial thickness measures can vary in the reproductive age group with maximum thickness in the secretory phase and up to 5 mm in the postmenopausal age group. The junctional zone surrounding the endometrium is composed of compact myometrial cells and appears homogeneously dark on the T2-weighted images and measures up to11–12 mm in the reproductive age group. The endometrial–myometrial interface is smooth and any interface irregularity may raise a suspicion for pathology. On the dynamic post-contrast T1-weighted images, an early subendometrial band (junctional zone) of enhancement is identified, which corresponds to the endometrial–junctional zone interface. In postmenopausal women, the junctional zone undergoes morphological changes resulting in poor delineation of the junctional zone–myometrial junction. The outer myometrium surrounding the junctional zone appears intermediate in T2 signal intensity.
The uterine cervix consists of the portio, which protrudes into the vagina, and a supravaginal part. The endometrial cavity continues into a spindle-shaped cervical canal at the level of the internal os. The cervical canal extends between from the internal os superiorly and external os inferiorly. Surrounding the cervical canal a hyperintense zone composed of glandular tissue and mucus glands is identified. The vaginal fornices delineate the external os. Squamous cells cover the epithelial surface of the portio, and, with age, they cover the columnar cells of the endocervical gland. This is a critical transitional zone called the squamocolumnar junction (SCJ), which represents the site of origin for carcinoma of the cervix (Fig. 27.6). The low T2 signal fibromuscular stromal ring surrounds the hyperintense zone and represents the inferior continuation of the junctional zone.
Pathological classification of uterine corpus abnormalities is described below (Algorithm 27.1).
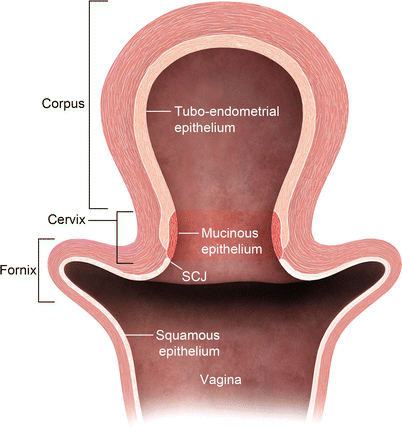
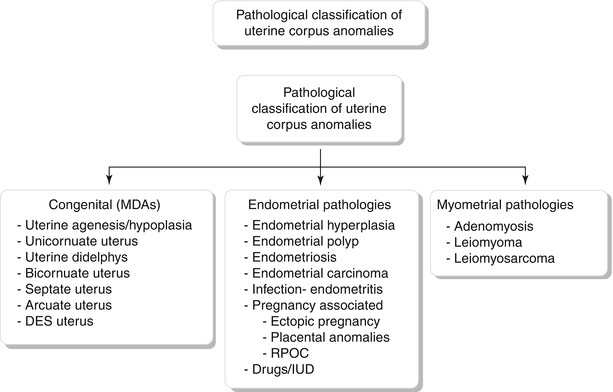

Fig. 27.6
Cervical transitional zone, which represents the critical squamocolumnar region (SCJ) which is the site for origin of carcinoma cervix. Drawing of the uterus and vagina. The cervix consists of two different types of epithelium: squamous epithelium and glandular epithelium. Squamous cells cover the epithelial surface continuing from the vagina, and columnar cells cover the glandular epithelium of the endocervical gland, which produces mucin. With age, squamous cells grow back to cover the columnar cells. This transitional area is the squamocolumnar junction (SCJ)

Algorithm 27.1 Pathological classification of uterine corpus anomalies
Congenital Anomalies
Mullerian duct anomalies (MDA) are a group of developmental disorders of the internal female genital tract, which represent a continuum of anomalies rather than distinct entities, with few of the complex anomalies having overlapping features. MDAs result from nondevelopment, defective fusion, or failure of resorption of the mullerian ducts [2]. Urinary tract anomalies are associated with MDAs in up to 30 % of cases [3]. Most of these anomalies are clinically asymptomatic; however, if obstruction occurs, patients may present with an abdominal mass, primary amenorrhea, or infertility. MDAs are associated with abnormal myometrial development resulting in weakness and thinning. Other complications include poor reproductive outcome, ectopic pregnancy, and an increased risk for the development of endometriosis. Initial diagnosis may be made at the time of delivery or during evaluation for infertility or recurrent miscarriages. Imaging forms a vital crux in the diagnostic evaluation and most often MDAs are diagnosed using USG and hysterosalpingography. Presently, MR is the modality of choice to evaluate MDAs.
MDAs have been classified by the American Society of Reproductive Medicine based on the anatomic findings (Table 27.1) (Figs. 27.7, 27.8, 27.9, 27.10, 27.11, 27.12, and 27.13) [4, 5]. However, vaginal anomalies are not included in this classification, and various other alternative classification systems have been offered.
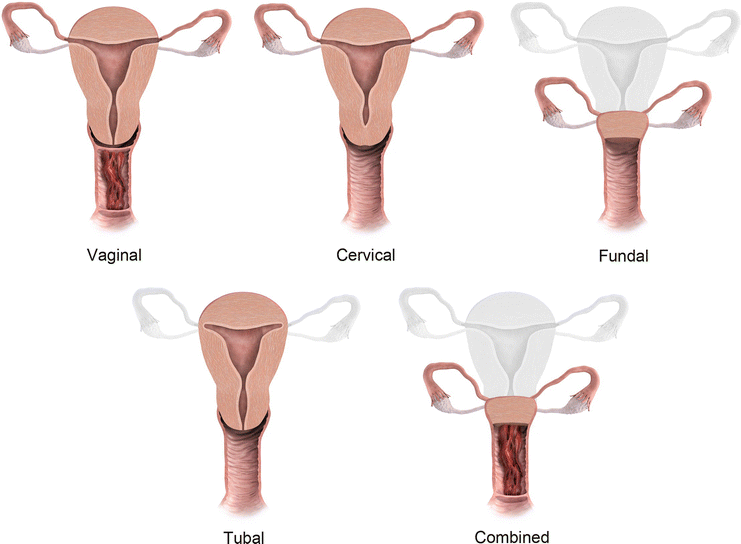
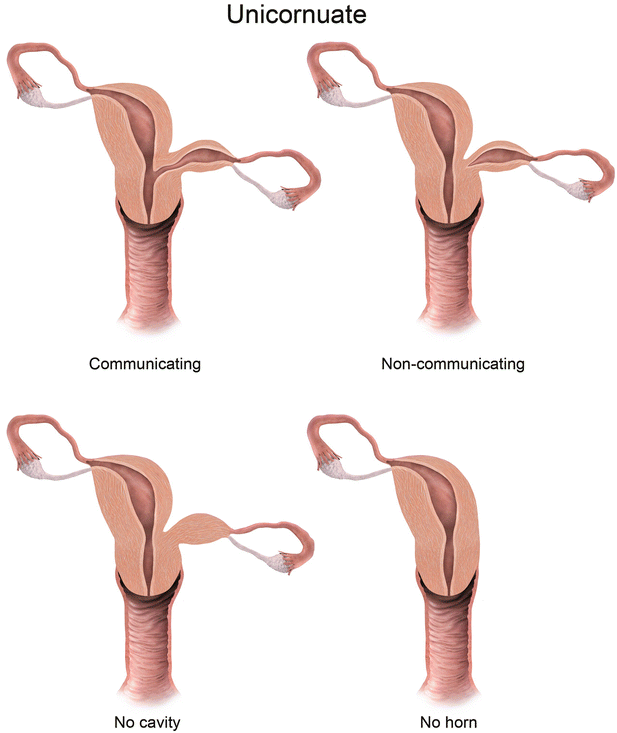
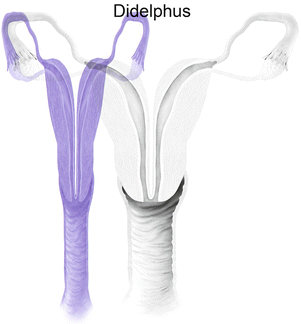
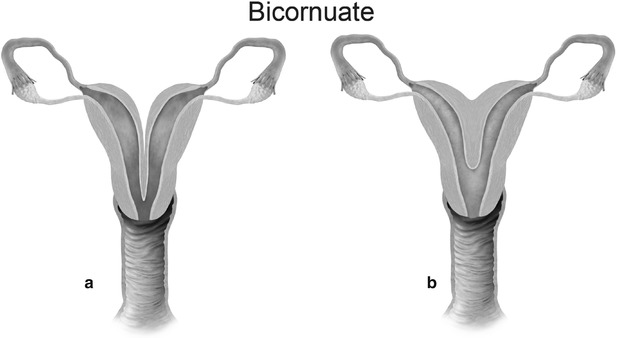
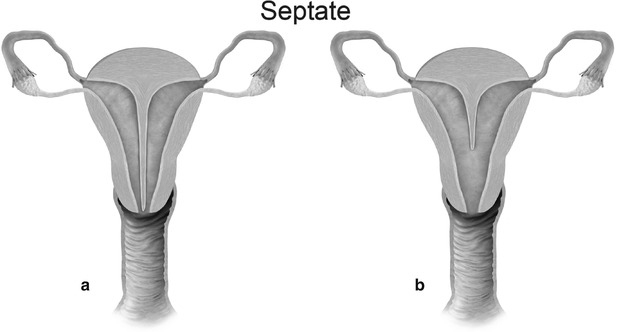
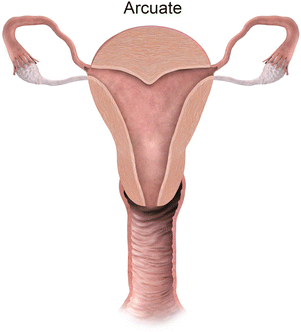
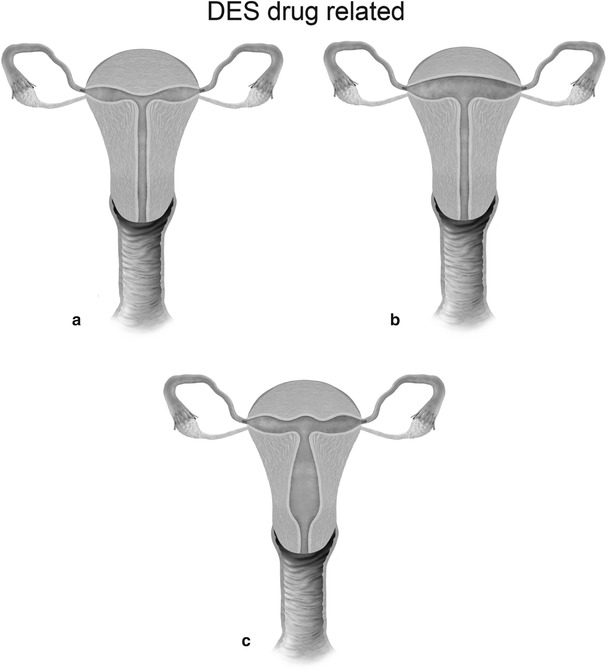

Fig. 27.7
Mullerian anomaly I: hypoplasia/agenesis. Various forms of hypoplasia are identified, including vaginal, cervical, fundal, tubal, or combined forms

Fig. 27.8
Mullerian anomaly II: unicornuate uterus

Fig. 27.9
Mullerian anomaly III: uterine didelphys

Fig. 27.10
Mullerian anomaly IV: bicornuate uterus complete (a) and partial (b)

Fig. 27.11
Mullerian anomaly V: septate uterus complete (a) and partial (b)

Fig. 27.12
Mullerian anomaly VI: arcuate uterus

Fig. 27.13
Mullerian anomaly VII: DES related-T-shaped uterus (a), T-shaped uterus with dilated horns (b) and uterine hypoplasia (c)
Table 27.1
Mullerian duct anomalies
ASM class | Percentage of MDAs | Description |
|---|---|---|
I | 5–10 | Mullerian agenesis or hypoplasia |
IA | Vaginal agenesis or hypoplasia (normal or abnormal uterus) | |
IB | Cervical agenesis or hypoplasia | |
IC | Fundal agenesis or hypoplasia | |
ID | Fallopian tube agenesis or hypoplasia | |
IE | Combined agenesis or hypoplasia (two or more from IA to ID) | |
II | 10–20 | Unicornuate uterus |
IIA | Rudimentary horn with an endometrial cavity communicating with uterus (single horn) | |
IIB | Rudimentary horn with an endometrial cavity not communicating with uterus | |
IIC | Rudimentary horn without an endometrial cavity | |
IID | No rudimentary horn | |
III | 5–20 | Uterine didelphys |
IV | 10 | Bicornuate uterus |
IVA | Complete bicornuate uterus (septum extends to internal/external os) | |
IVB | Partial bicornuate uterus (septum in fundal region) | |
V | 55 | Septate uterus |
VA | Complete septate uterus | |
VB | Partial septate uterus | |
VI | Arcuate uterus | |
VII | 69 | DES-related uterine anomalies |
VIIA | T-shaped uterus | |
VIIB | T-shaped uterus with dilated horns | |
VIIC | Uterine hypoplasia |
Class I: Mullerian agenesis (Fig. 27.14) and hypoplasia (Fig. 27.15) represent the most severe form of MDA [6]. Mayer-Rokitansky-Kuster-Hauser syndrome consists of complete vaginal agenesis with varying degree of uterine corpus and cervical anomalies and is commonly associated with renal or vertebral anomalies. Occasionally, a rudimentary functioning or nonfunctioning mullerian remnant may be seen. The principal role of MR imaging in class I anomalies is to differentiate between uterine agenesis and hypoplasia.
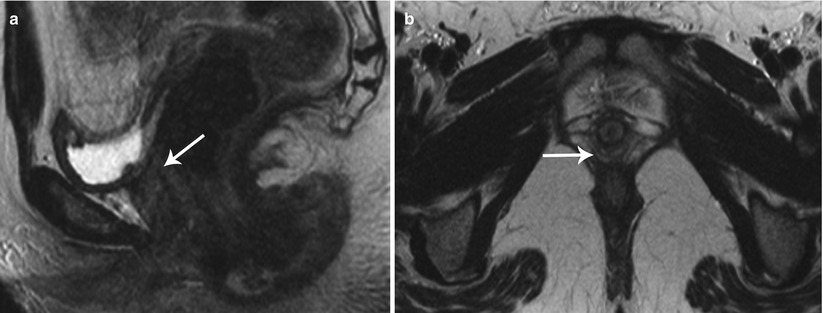
Fig. 27.14
Uterine agenesis. T2-weighted sagittal (a) and axial (b) show complete mullerian agenesis with absence of the uterus, cervix, and vagina (white arrows)
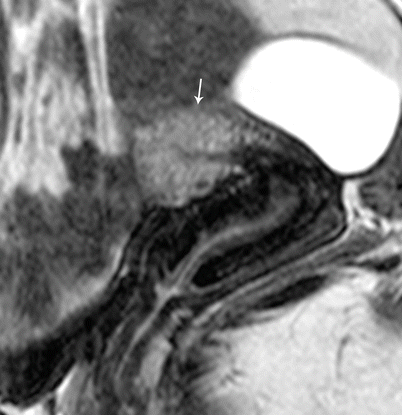
Fig. 27.15
Uterine hypoplasia. T2-weighted sagittal image shows uterine hypoplasia (white arrow), with a small uterine corpus, absence of an endometrial cavity, and poor zonal differentiation
Class II: Unicornuate uterus represents failure of one mullerian duct to reach the urogenital sinus, which results in a single-horned uterus opening into a normal vagina. The rudimentary horn may either be cavitary or noncavitary [7]. A cavitary rudimentary horn may either be communicating (connection with the contralateral horn) or noncommunicating. On MR imaging, a unicornuate uterus appears as a small curved structure with a pointed apex (Fig. 27.16). Presence of functioning endometrial tissue in the rudimentary horn results in a blood-filled distended cavity, which needs surgical intervention. On MR imaging, functioning endometrial tissue appears as a hyperintense stripe in the midst of rudimentary tissue, and patient needs surgery to avoid future endometriosis. If the MR findings are equivocal and clinical suspicion persists, laparoscopic evaluation may be performed.
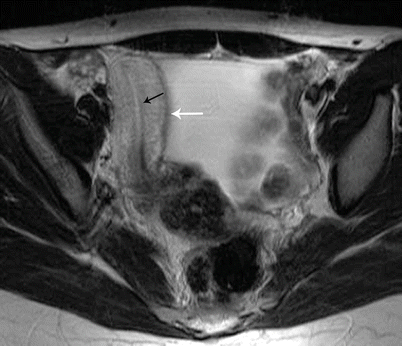
Fig. 27.16
Unicornuate uterus. T2-weighted axial image shows a cavitary unicornuate uterus (white arrow) with a functioning T2 hyperintense endometrial lining (black arrow)
Class III: Uterus didelphys is a symmetric anomaly representing failure of fusion of both paramesonephric ducts, resulting in two completely separate uterine cavities and cervix [8]. Uterine didelphys is associated with a complete or partial longitudinal vaginal septum [6, 9]. Some patients may present with a unilateral hemivaginal septum [6, 9], which is commonly associated with ipsilateral renal agenesis [3, 6]. MR imaging demonstrates two widely divergent uterine horns, with a deep fundal cleft, two separate cervixes, and an upper vaginal septum (Fig. 27.17). Each cavity has normal functioning endometrial lining and myometrial tissue.
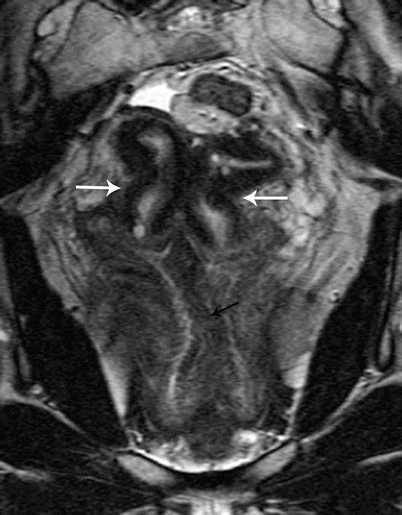
Fig. 27.17
Uterine didelphys. T2-weighted coronal image shows uterine didelphys, with two completely separate uterine cavities and cervix (white arrows), and a longitudinal vaginal septum with two vaginal cavities (black arrows)
Class IV: Bicornuate uterus is a symmetric anomaly, representing incomplete fusion of the mullerian ducts at the level of the fundus, resulting in two symmetric cornua with communication of the endometrial cavities, mostly at the level of the isthmus. The septum may extend to either the internal or the external os resulting in a unicollis or bicollis uterus. When the septum is confined to the fundus, the entity represents a partial bicornuate uterus [10]. The length of the septum influences rates of spontaneous abortion and preterm delivery. MR imaging demonstrates two separate uterine horns with an intervening deep notch, measuring more than 1 cm in length (Fig. 27.18) [11, 12]. Each horn has normal functioning endometrial lining and myometrial tissue. The intercornual distance measures 4 cm or more in bicornuate uterus. Coexistent vaginal septum may result in obstruction and unilateral or bilateral hematometrocolpos (Fig. 27.19).
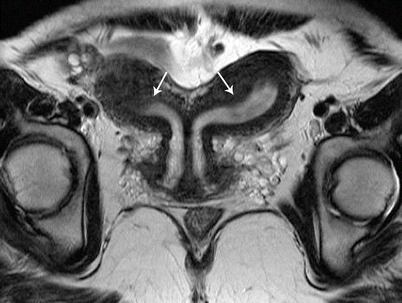
Fig. 27.18
Bicornuate uterus. T2-weighted coronal image shows bicornuate uterus with two completely separate uterine cavities and cervix (white arrows)
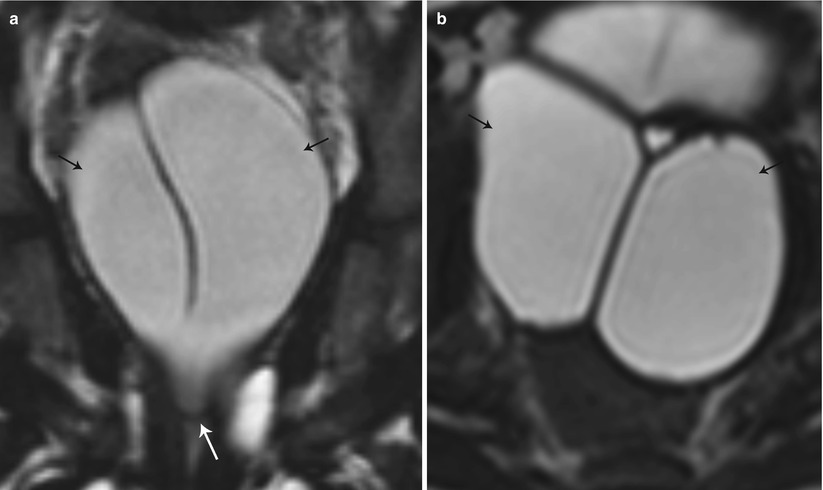
Fig. 27.19
Partial bicornuate uterus with transverse vaginal septum. T2-weighted coronal (a) and axial (b) images show bilateral hematometra (black arrows) with a transverse vaginal septum (white arrow)
Class V: Septate uterus represents incomplete resorption of the septum following complete fusion of the mullerian ducts [6]. This is the most common MDA, with the septum located in the midline fundal region, and is associated with poor obstetrical outcome. A complete septum extends to the internal or external os and a partial septum does not reach the internal os [13]. MR imaging demonstrates a normal-sized uterus, with a partial or complete division of a small endometrial cavity by a solid mass in the fundus, which is isointense to the myometrium (Fig. 27.20). The outer contour of the uterine fundus is convex, with the intercornual distance measures less than 4 cm in a septate uterus. Histologically, the septum may either be fibrous or muscular. On MR imaging it is essential to measure the thickness of the septum, prior to septoplasty or metroplasty.
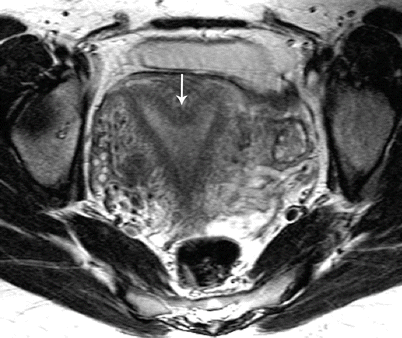
Fig. 27.20
Septate uterus. T2-weighted axial image shows a septate uterus, with a septum in the mid fundal region (white arrow)
Class VI: Arcuate uterus represents near complete resorption of the uterovaginal septum, with some authors considering this entity a normal variant [9], as it is clinically asymptomatic. MR imaging depicts a normal external contour, with a small indentation of the fundal endometrial canal, and no division of the horns.
Class VII: Diethylstilbestrol is a banned nonsteroidal estrogen, which produced congenital non-obstructive anomalies of the uterine corpus, cervix, and vagina and was also associated with clear cell carcinoma of vagina. In the present day scenario, these anomalies are extremely rare.
Vaginal septal anomalies are classified based on the system developed by Digwani and Falcone (Fig. 27.21) [14]. These are classified into three subtypes – transverse vaginal septum (type I), longitudinal vaginal septum (type II), and stenosis (type III). Transverse vaginal septum represents a vertical fusion defect due to failure of resorption of the tissue between the vaginal plates [15]. Transverse septa are commonly located in the upper vagina in about 46 % of cases; however, these septa can also be identified in the mid (40 %) or lower (14 %) vagina [16]. Patients are usually asymptomatic, presenting only at the onset of menses or later in life due to dyspareunia. MR imaging is used to determine the thickness of the septum and differentiate a high septum from congenital absence of the cervix. Longitudinal vaginal septum occurs either secondary to failure of fusion of the mullerian ducts or incomplete resorption of the vaginal septum [17]. Longitudinal vaginal septa usually occurs in combination with uterine anomalies, mostly uterus didelphys. On MR imaging longitudinal septum appears as a thin, dark T2 signal structure separate from the high T2 signal vaginal mucosa.
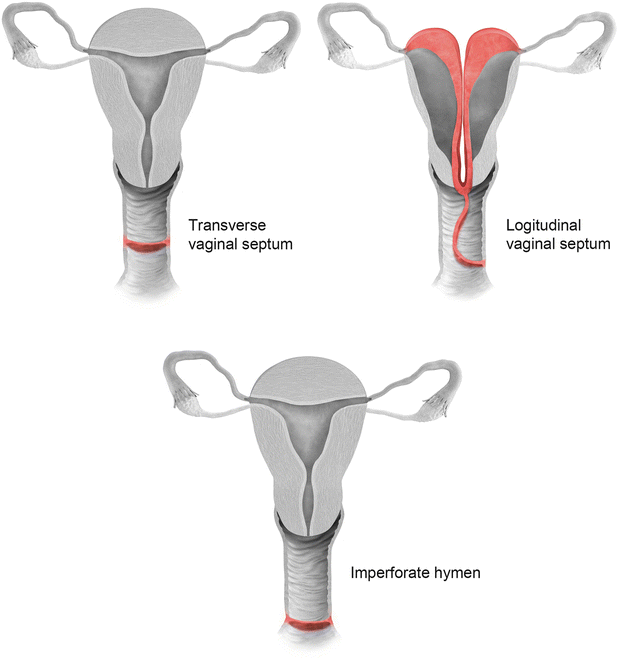

Fig. 27.21
Classification of vaginal anomalies
Endometrium
Endometrium is the innermost glandular lining of the uterus. The endometrium demonstrates a wide spectrum of normal morphological appearances during the prepubertal years, menarche, and postmenopausal years. Hence, it is pertinent to evaluate and interpret the endometrial appearance in lieu of the patient’s age, stage of the menstrual cycle, coexistent medical illnesses, and drug therapy. At birth, the endometrium appears as a thin line, with or without distension of the endometrial canal [18]. On achieving puberty, the endometrium simulates the adult (premenopausal) appearance. The premenopausal endometrium undergoes cyclical regeneration, measuring 1–4 mm in thickness during menstruation, 5–7 mm during the proliferative phase, up to 11 mm during the late proliferative phase, and 7–16 mm in the secretory phase [19]. In postmenopausal patients, endometrial thickness varies based on the clinical setting, with an upper limit of 4 mm in asymptomatic patients, 5 mm in symptomatic patients, and 8 mm in patients on hormonal therapy. Endometrial thickness is measured on a midsagittal image across the endometrial cavity. On USG, the premenopausal endometrium appears as an echogenic stripe, measured from echogenic border to echogenic border across the endometrial cavity. During the late proliferative phase, the endometrium has a trilayered appearance, which typically disappears 48 h after ovulation. On MR images, the normal premenopausal endometrium appears as a uniform high T2 signal intensity stripe. The normal postmenopausal endometrium is atrophic and appears as a homogeneous smooth endometrium, without focal thickening, measuring less than 5 mm in thickness [20, 21].
Any significant deviation in an established menstrual cycle may be considered as abnormal bleeding. Menstrual irregularities include metrorrhagia, menometrorrhagia, polymenorrhea, oligomenorrhea, menorrhagia, and hypomenorrhea. Histology constitutes the definitive diagnosis; however, imaging plays a key role in screening and identifies patients who require more invasive workup with hysteroscopy or excisions. A wide spectrum of disorders is known to produce abnormal uterine bleeding, and these are typically classified into three subgroups: premenopausal, pregnancy related/peripartum, and postmenopausal (Algorithm 27.2). In this chapter we will discuss in detail the algorithmic approach to pre- and postmenopausal bleeding (Algorithm 27.3 and 27.4) and their various etiologies. Only few peripartum abnormalities will be briefly discussed in this chapter.

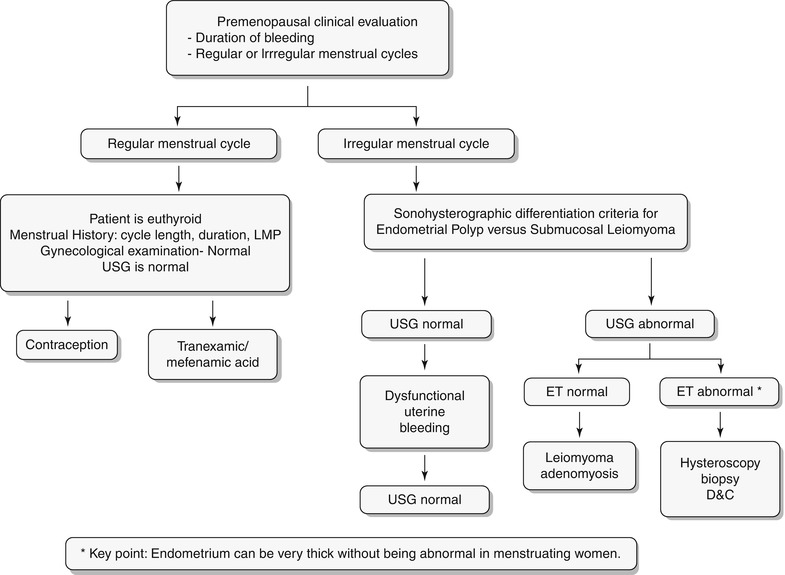
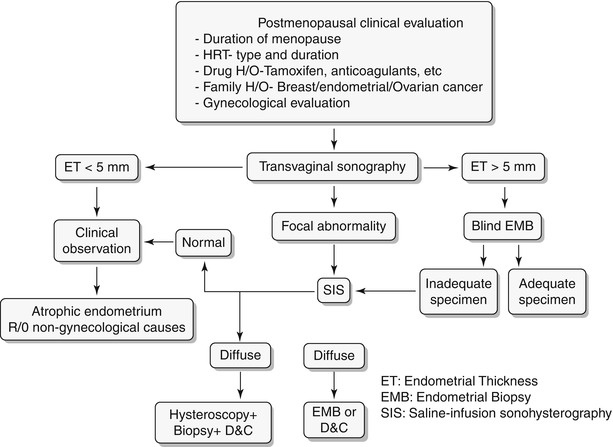

Algorithm 27.2 Classification of abnormal vaginal bleeding

Algorithm 27.3 Clinical algorithmic approach to evaluation of premenopausal bleeding

Algorithm 27.4 Clinical algorithmic approach to evaluation of postmenopausal bleeding

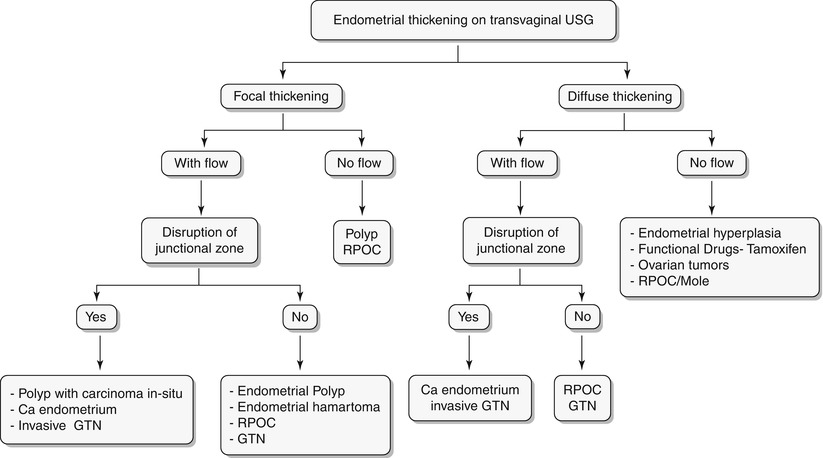
Algorithm 27.5 Algorithmic approach to endometrial thickening on transvaginal USG
Endometrial Hyperplasia
Endometrial hyperplasia is defined as abnormal proliferation of endometrial stroma and glands, which varies from glandular atypia to frank neoplasia. Various forms of endometrial hyperplasia exist, including cystic, adenomatous, and atypical; however, these forms present either as diffuse and less commonly focal endometrial thickening (Algorithm 27.5), though this is not a widely accepted criterion. Algorithm 27.6 describes sonohysterographic criteria to differentiate endometrial hyperplasia from endometrial carcinoma. However, imaging alone cannot reliably differentiate between endometrial hyperplasia and carcinoma (Fig. 27.22). Endometrial hyperplasia has a nonspecific appearance on both USG and MR imaging; hence, if any clinical suspicion for malignancy exists, biopsy of the focal endometrial abnormality should be performed.
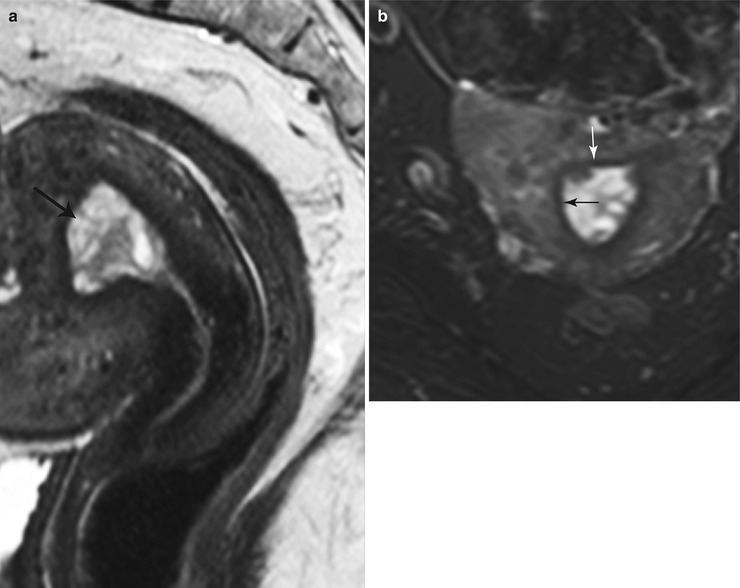
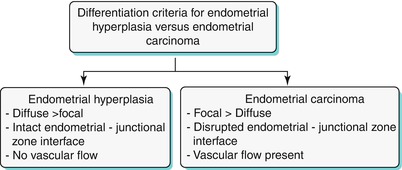
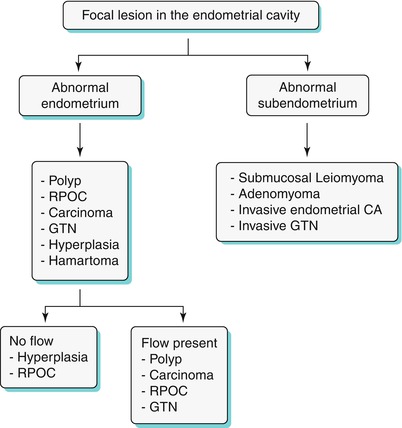

Fig. 27.22
Endometrial hyperplasia. T2-weighted sagittal (a) and T2-weighted fat-suppressed axial (b) images show diffuse cystic endometrial thickening (black arrow) with a smooth endometrial–junctional zone interface (white arrow)

Algorithm 27.6 Sonohysterographic criteria to differentiate endometrial hyperplasia from endometrial carcinoma

Algorithm 27.7 Algorithmic approach to a focal endometrial cavitary lesion
Endometrial Polyp
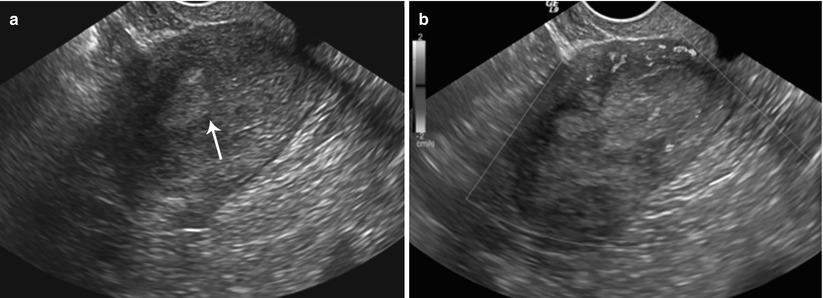
Endometrial polyps are benign nodular protrusions of the endometrial surface, consisting of irregularly distributed endometrial glands and stroma. Polyps may occur in isolation or in combination with endometrial hyperplasia; however, polyps are a more common cause of abnormal and often focal endometrial thickening than hyperplasia [22]. Algorithm 27.7 describes approach to a focal endometrial cavitary lesion. On USG, endometrial polyps may appear as naonspecific endometrial thickening or as focal masses within the endometrial canal. On sonohysterography, polyps appear as echogenic, smooth, intracavitary masses, with a vascular pedicle (Fig. 27.23) [23, 24]. Submucosal fibroids are the commonest differentials on sonohysterography and appear as broad-based lesions with posterior acoustic shadows arising from the myofibrous interfaces (Fig. 27.24 and Algorithm 27.8). On MR imaging, polyps appear generally as heterogeneous high T2 signal intensity masses with or without distension of the endometrial cavity. Smaller polyps may not be visible. Typical MR features include the presence of a central low T2 signal intensity fibrous core and multiple smooth-walled, variable-sized, well-defined intratumoral cysts and lattice-like enhancement on the T1-w images (Fig. 27.25) [25]. Based on these MR findings alone, accurate differentiation from endometrial carcinoma is not possible. Myometrial invasion represents the sole important finding that differentiates endometrial carcinoma from a polyp.
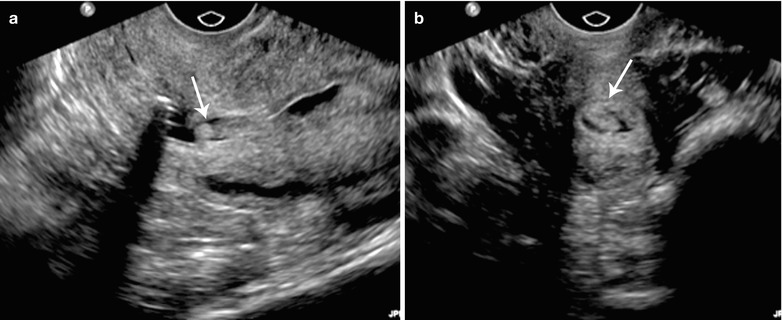
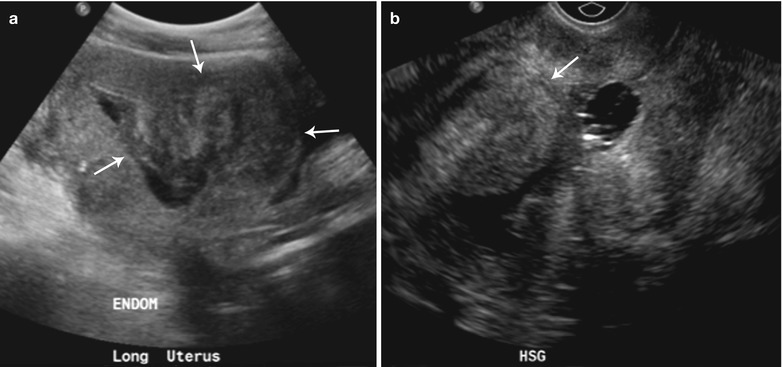
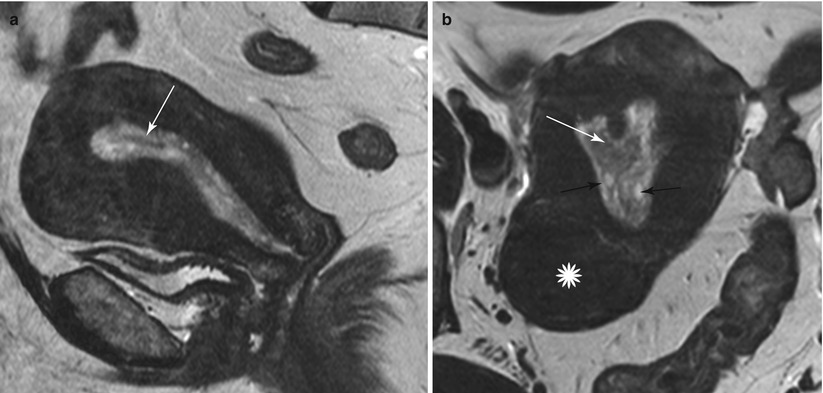
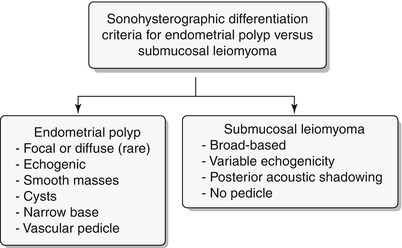

Fig. 27.23
Endometrial polyp. 45-year-old female with abnormal vaginal bleeding. Sonohysterogram (a and b) revealed two soft tissue projections in the endometrial canal, one from anterior wall with narrow stalk and other from posterior wall with broader stalk, representing endometrial polyps (arrows)

Fig. 27.24
Submucosal fibroid 47-year-old female with a history of fibroids, presenting for evaluation of the endometrium. Sonohysterogram (a and b) shows single, narrow-based, echogenic polypoid lesion, representing an endometrial polyp arising from posterior endometrial wall with several tiny cysts (arrow)

Fig. 27.25
Endometrial polyp. T2-weighted sagittal (a) and axial (b) shows the presence of a central low T2 signal intensity fibrous core arising from the fundus (white arrow), with multiple well-defined T2 hyperintense intratumoral cysts (black arrow). Note an intramural fibroid (asterisk)

Algorithm 27.8 Sonohysterographic criteria to differentiate endometrial polyp from submucosal leiomyoma
Endometriosis
Endometriosis is a common disorder afflicting women in the reproductive age group and is defined as the presence of functional endometrial glands and stroma outside (ectopic) the uterine cavity [26]. The ectopic endometrial tissue responds to hormonal stimulation during menstrual cycle, leading to cyclical hemorrhage. Occasionally, endometriosis is detected in adolescent girls due to underlying obstructive mullerian duct anomalies [27] and in postmenopausal women who are on estrogen replacement therapy [28]. Controversy surrounds the likely etiopathogenesis of endometriosis. Various theories have been proposed, including metastatic, metaplastic, and induction; however none of these theories explain in entirety the likely mechanism. Metastatic theory is most widely accepted and explains the presence of endometriotic implants along peritoneal reflections and its spread to distant sites via venolymphatics. Metaplastic theory suggests the transformation of peritoneal lining into the endometriotic tissue and explains the presence of implants in the absence of functioning native endometrial lining. Lastly, induction represents an amalgam of the first two theories, with the transformation of mesenchyme into functioning endometrium due to inducing agents. Other theories which are under investigation include an impaired immune response and the role of hormonal and growth factors.
Endometriosis encompasses the following entities: endometriomas, peritoneal implants, deep pelvic (extraperitoneal) endometriosis, and adhesions. The most common locations include the ovaries, pelvic peritoneum, deep subperitoneal lesions, intestines, and urinary system. Peritoneal implants are usually asymptomatic (Fig. 27.26). On the contrary, deep pelvic endometriosis is associated with infertility and present with pelvic pain, dysmenorrhea, dyspareunia, urinary and gastrointestinal symptoms, and radiating pain due to involvement of the lumbosacral plexus. Endometriosis is staged using the Revised Classification of Endometriosis published by the American Fertility Society (Table 27.2) [29]. Based on the staging system, patients are categorized as having minimal, mild, moderate, and severe disease. However, the staging does not seem to correlate with the clinical symptoms and has not led to the formulation of standardized therapeutic options [30].
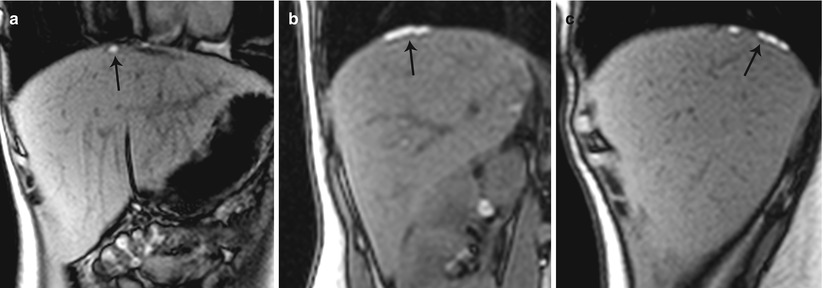
Table 27.2
Endometriosis staging system by the American Fertility Society
(A) Endometrial implants | ||||
Size | ||||
Location | Depth of penetration | <1 cm | 1–3 cm | >3 cm |
Peritoneum | Superficial | 1 | 2 | 4 |
Deep | 2 | 4 | 6 | |
Ovary | Right superficial | 1 | 2 | 4 |
Right deep | 4 | 16 | 20 | |
Left superficial | 1 | 2 | 4 | |
Left deep | 4 | 16 | 20 | |
(B) Obliteration of the posterior cul-de-sac | ||||
Degree of Obliteration | Score | |||
Partial | 4 | |||
Complete | 40 | |||
(C) Adhesions | ||||
Amount of surface involvement | ||||
<1/3 | 1/3–2/3 | >2/3 | ||
Location | Appearance | Enclosure | Enclosure | Enclosure |
Ovary | Right filmy | 1 | 2 | 4 |
Right dense | 4 | 8 | 16 | |
Left filmy | 1 | 2 | 4 | |
Left dense | 4 | 8 | 16 | |
Fallopian tube | Right filmy | 1 | 2 | 4 |
Right dense | 4 | 8 | 16 | |
Left filmy | 1 | 2 | 4 | |
Left dense | 4 | 8 | 16 | |

Fig. 27.26
Peritoneal endometrial implants in the subdiaphragmatic region. T1-weighted out-of-phase coronal (a) and sagittal (b, c) images reveal hyperintense plaque-like subdiaphragmatic endometrial implants (black arrows)
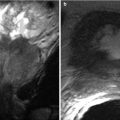
Endometriomas appear as hemorrhagic unilocular or multilocular cystic lesions of the ovary, typically called chocolate cysts. Multilocular endometriomas are separated by thin or thick septations. On USG, endometriomas appear as homogeneous hypoechoic lesions with low-level internal echoes [31]. Occasionally, these may appear anechoic and simulate functional ovarian cysts. Echogenic foci along the cyst wall represent an important predictor of endometriomas [31]. Features more commonly attached to cystic ovarian neoplasms such cyst wall thickening and mural nodularity may also be associated with endometriomas [31]. On MR, endometriomas appear as solitary or multiple hyperintense cysts on T1-weighted images, with evidence of shading on the T2-weighted images (Fig. 27.27) [32, 33]. Shading on the T2-weighted images depends on the concentration of blood products [32]. The differentials include hemorrhagic cyst, dermoid, and cystic ovarian neoplasm.
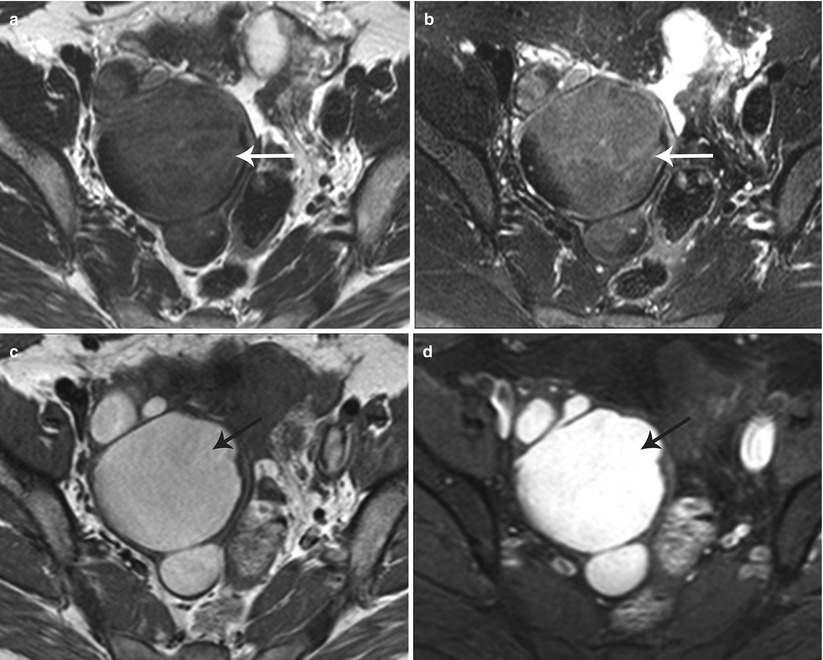

Fig. 27.27
Bilateral ovarian endometriomas. T2-weighted axial images (a, b) show bilateral complex cystic adnexal lesions with T2 shading effect (white arrows). T1-weighted (c) and pre-gadolinium T1-weighted fat-suppressed (d) axial images show hemorrhagic contents within the adnexal lesions (black arrows)
Deep pelvic endometriosis is defined as subperitoneal invasion by endometriotic lesions that exceed 5 mm in depth. Typically it affects extraperitoneal sites, such as the fibromuscular pelvic support structures, vagina, bowel, and urinary tract. Deep endometriotic implants are separated according to their anatomic location in the anterior cul-de-sac, posterior cul-de-sac, or pelvic sidewall (Fig. 27.28) [34]. Anterior cul-de-sac lesions are identified within the vesicouterine pouch, vesicovaginal septum, bladder (detrusor nodule), and ureter. Posterior cul-de-sac lesions include retroperitoneal and dependent intraperitoneal lesions. Retroperitoneal posterior cul-de-sac lesions are classified as rectovaginal (type I), posterior forniceal (type II), and hourglass (type III) lesions (Fig. 27.29) [35]. Posterior forniceal lesions are identified in approximately 65 % of cases, followed by hourglass lesions and rectovaginal septum lesions in 25 % and 10 % of cases, respectively [35]. Hourglass lesions have the greatest predilection to infiltrate the rectal wall [35].
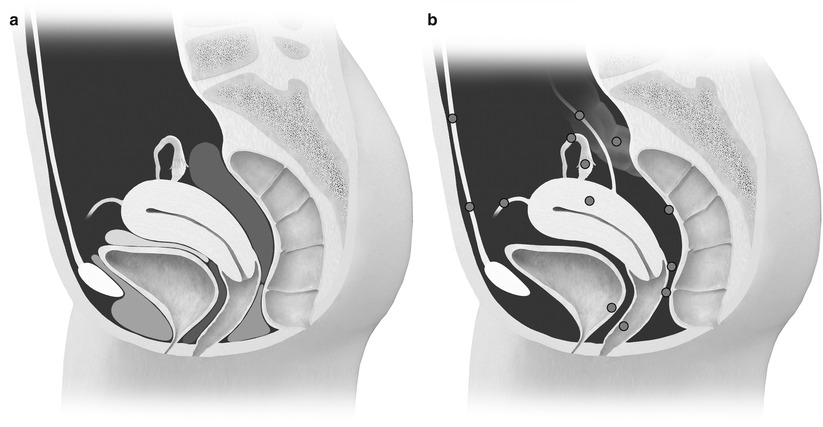
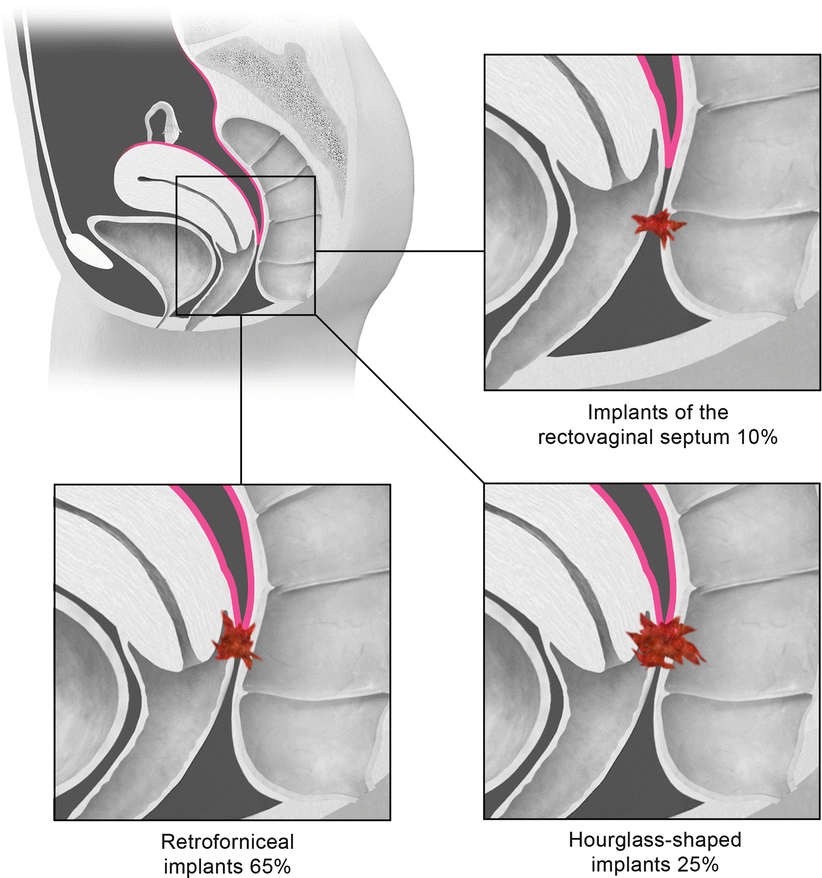

Fig. 27.28
(a) Normal anatomy of the anterior and posterior cul-de-sac compartments. Anterior cul-de-sac: prevesical space (green), vesicouterine pouch (yellow), and vesicovaginal septum (blue). Posterior cul-de-sac: rectovaginal septum (orange) and rectouterine pouch (red). (b) Possible locations of endometriosis

Fig. 27.29
Classification of posterior cul-de-sac lesions into rectovaginal (type I), posterior forniceal (type II), and hourglass (III) lesions
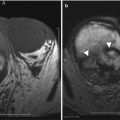
Anterior cul-de-sac lesions, which involve the ureter and bladder, have more advanced stages of the disease. On MR, these appear as low T2 signal nodular lesions with few T1 hyperintense foci obliterating the anterior cul-de-sac due to extensive adhesions associated with acute anteflexion of the uterus. Vesical endometriosis presents as an extrinsic low T2 signal intensity serosal deposit or as intrinsic lesion infiltrating the bladder wall (Fig. 27.30) [31]. Rarely, mucosal invasion is identified and MR can depict disease in patients with normal cystoscopic findings [36]. Ureteral endometriosis is typically extrinsic; however, deep infiltrative lesions may also occur. Typically, these are low T2 signal intensity lesions infiltrating the adventitia and adjacent periureteric tissues [37], resulting in ureteral scarring, retraction, and obstruction causing proximal hydroureteronephrosis. Urethral endometriosis is rare, usually observed as a contiguous lesion arising from the bladder.
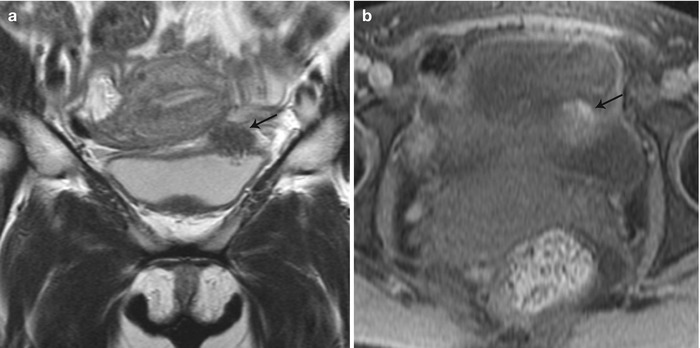

Fig. 27.30
Vesical endometriosis. T2-weighted coronal (a) image shows a low T2 signal implant in the bladder dome on the left (black arrow), which appears heterogeneously hyperintense on the pre-gadolinium T1-weighted fat-suppressed (b) axial image (black arrow)
Posterior cul-de-sac lesions typically present as low T2 signal enhancing nodular lesions obliterating the cul-de-sac and retracting the posterior fornix, associated with acute uterine retroversion or rectal retraction (Fig. 27.31). Uterosacral ligaments are typically involved with retrocervical lesions and present on MR as bilateral or asymmetric low T2 signal thickening, primarily involving the proximal portion of the ligaments [36]. Such lesions extend into the vaginal fornices or rectal wall. Rectosigmoid is the commonest bowel segment involved in deep pelvic endometriosis (Fig. 27.32). Implants are usually superficial but occasionally infiltrate the muscle causing rectal wall thickening, severe fibrosis, retraction, adhesions, bowel strictures, and obstruction.
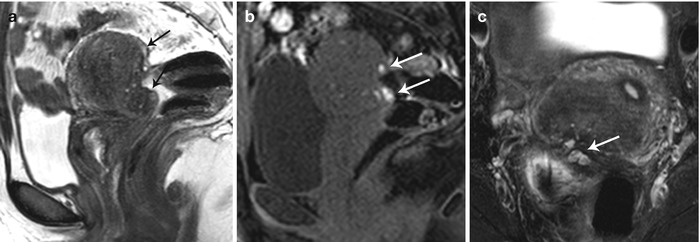
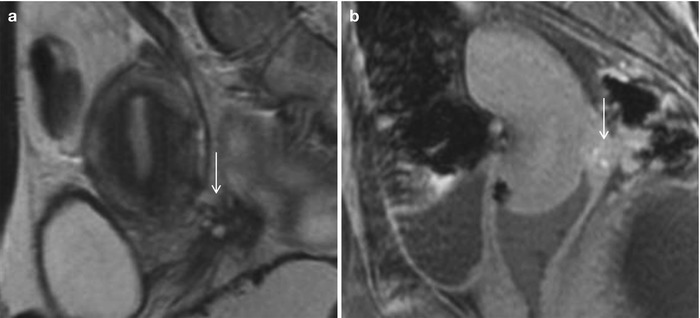

Fig. 27.31
Posterior cul-de-sac endometriosis with uterine serosal implants. T2-weighted sagittal (a), pre-gadolinium T1-weighted fat-suppressed sagittal (b) and T2-weighted fat-suppressed axial images show hemorrhagic implants on the posterior uterine serosal surface and retrocervical region (white arrows) causing mild uterine retroversion with retraction of the posterior fornix and anterior rectal wall (black arrows)

Fig. 27.32
Rectovaginal endometriotic implant: T2-weighted sagittal image (a) shows cluster of microcysts (arrow) along the rectovaginal septum (involvement of posterior forniceal wall and anterior rectal wall) with surrounding puckering/scarring. Fat saturated T1-weighted image (b) shows hyperintensity corresponding to microcysts (arrow) consistent with foci of hemorrhages or proliferating endometrium in a case of endometriosis
Lateral pelvic wall implants arise from the ovary or adjacent peritoneal disease, typically involving the ureter with a reported prevalence of 0.01–1 % (Fig. 27.33) [38] or the lumbosacral plexus causing cyclical sciatica (Fig. 27.34). MR depicts deep pelvic endometriosis as either small infiltrative implants, solid deep lesions in the anterior or posterior cul-de-sac, or visceral implants. Other unusual sites of involvement include small-bowel loops, vagina, and ischioanal fossa.
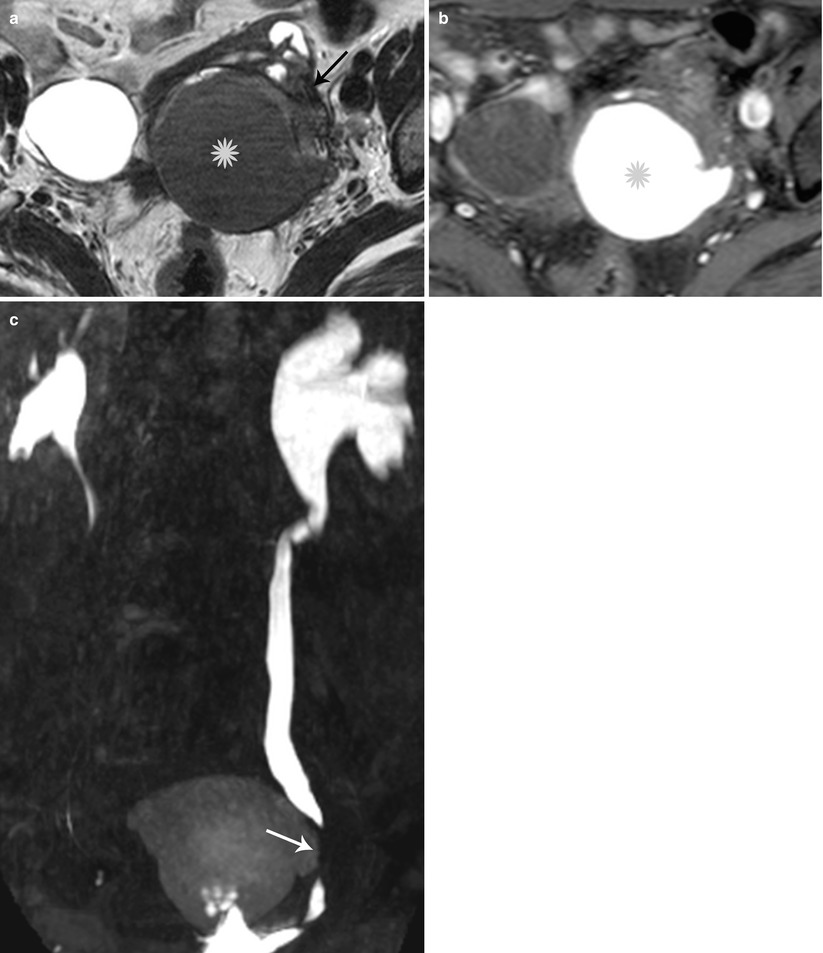
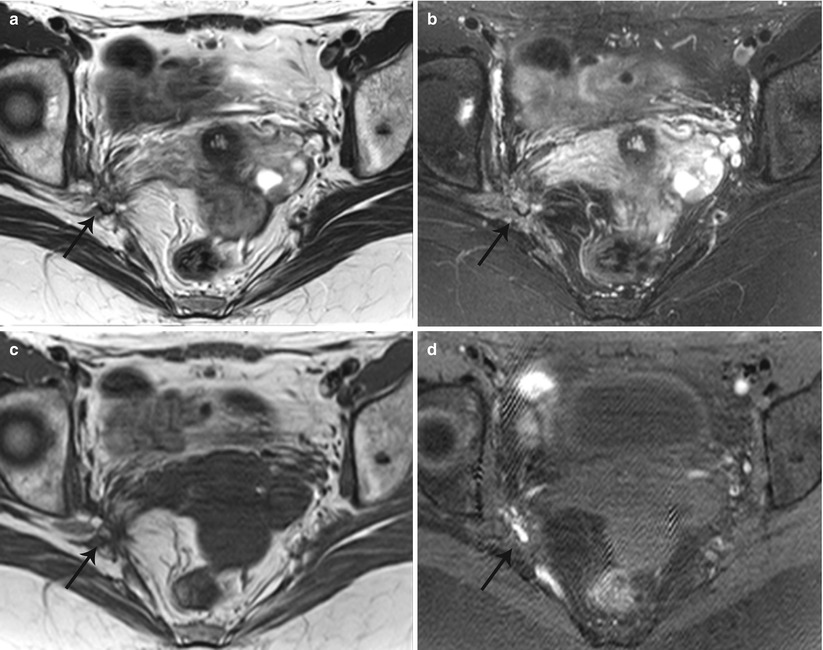

Fig. 27.33
Lateral pelvic wall endometriotic implant with ureteric involvement. Axial T2-weighted (a), axial pre-gadolinium fat-suppressed T1-weighted (b), and coronal fat-suppressed T2-weighted images (c) show a large left ovarian endometrioma (yellow asterisks) with low T2 signal fibrosis in the lateral pelvic wall (black arrow), with entrapment and infiltration of the left ureter (white arrow) causing hydroureteronephrosis

Fig. 27.34
Lateral pelvic wall endometriotic implant with right lumbosacral plexus involvement. T2-weighted axial (a), T2-weighted fat-suppressed axial (b), pre-gadolinium T1-weighted axial (c), and pre-gadolinium T1-weighted fat-suppressed axial (d) show an ill-defined plaque-like hemorrhagic lesion with localized fibrosis (black arrows) involving the right lumbosacral plexus adjacent to the greater sciatic foramen
Endometrial Carcinoma
Endometrial carcinoma is the most common gynecological malignancy and the fourth most frequent cancer in women worldwide [39]. In 2011, an estimated 46,470 newly diagnosed cases and 8,129 deaths are expected in the United States [40]. The prognostic factors linked with improved survival rates include the FIGO stage at initial diagnosis, depth of myometrial invasion, and histological grade, all of which strongly correlate with the presence of lymphovascular invasion and nodal involvement [41]. Five-year survival status in patients with endometrial carcinoma range between 95.8 % for stage I disease and 15.9 % for stage IV disease [40]. Over the last decade, early diagnosis primarily due to the advent of high-resolution MR techniques and improved treatment options have resulted in reduced mortality and improved survival rates. The risk factors for developing endometrial carcinoma include high levels of and prolonged exposure to estrogen, endometrial hyperplasia, obesity, nulliparity, infertility, tamoxifen usage, polycystic ovarian disease, and coexistent ovarian carcinoma.
Surgery is the treatment of choice in noninvasive or early endometrial cancer and consists of a combination of total hysterectomy with bilateral oophorectomy and peritoneal washing. In early stage disease, it may be important to differentiate cancer confined to endometrium versus cancer spreading to inner myometrium, due to the reported preference of hormonal treatment for endometrium confined cancer in some patients [42]. It is also of equal concern to differentiate stage IA from IB disease, to assess the risk for lymph node metastases which is more with IB disease, and thereby to accurately identify patients at risk for recurrence and therefore requiring more radical surgery or neoadjuvant therapies. With the prevalence of the disease in elderly women with comorbid conditions and the increasing need to use minimally invasive or laparoscopic surgeries in such patients, it is even more pertinent to accurately stage these patients [43, 44]. In such a scenario, MR plays a vital role in presurgical assessment, may determine surgical approaches, and may also assess the need for alternative therapies to downstage lesions.
Endometrial carcinomas are divided into two classes, primarily differentiated on the basis of pathophysiology and long-term prognosis. Type I lesions are the commonest, representing approximately 80 % of all endometrial carcinomas, and are secondary to long-standing exposure to estrogen. Endometroid adenocarcinoma is the commonest histological subtype representing approximately 75–80 % of all endometrial cancers. Simultaneous occurrence of morphologically individual endometroid adenocarcinoma of the uterus and ovaries are the most frequently observed synchronous genital malignancies (Fig. 27.35). Less common histologies include adenosquamous and mucinous subtypes. Type II lesions are estrogen-independent lesions, which typically occur in the elderly women and include clear cell and papillary serous subtypes. The clear cell and papillary serous subtypes represent 15–20 % of all cases, are high-grade lesions with poor prognosis, and present with nodal spread even in the absence of myometrial invasion. Imaging techniques including MR cannot reliably differentiate between the various subtypes of endometrial carcinoma.
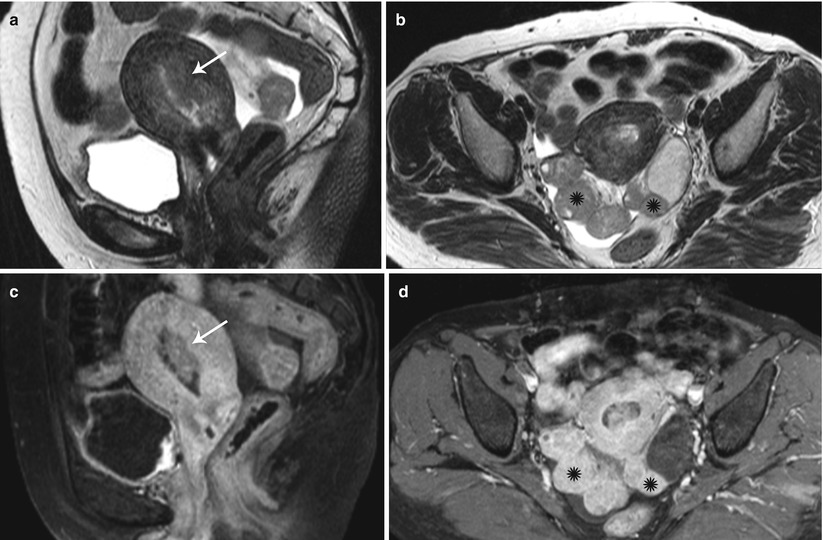

Fig. 27.35
Synchronous endometroid adenocarcinoma of uterus and bilateral ovaries. T2-weighted sagittal (a) and post-contrast T1-weighted fat-suppressed sagittal (c) images show an infiltrative stage Ib infiltrative endometrial carcinoma (white arrows). T2-weighted axial (b) and post-contrast T1-weighted fat-suppressed axial (d) images show bilateral ovarian solid enhancing lesions (black asterisks)
In a consensus statement, the Society of Radiologists in ultrasound has stated that transvaginal ultrasonography (US) is an acceptable modality for first-line assessment of postmenopausal bleeding [45], with an established endometrial thickness threshold at 5 mm, which confers 96 % sensitivity for detection of endometrial cancer. However, the US findings of endometrial carcinoma are nonspecific, with considerable overlap with endometrial hyperplasia and polyps. These findings include endometrial heterogeneity and irregular thickening, which in a setting of postmenopausal bleeding necessitates biopsy. Occasionally, polypoidal masses produce more diffuse and irregular endometrial thickening than hyperplasia (Fig. 27.36). A more specific but less common ultrasonographic sign is the presence of irregularity of the endometrium–myometrium interface, which suggests myometrial invasion. Unusually, the presence of hydro- or hematocolpos may raise concern for endometrial carcinoma, though this finding is more frequently associated with cervical carcinoma. The role of color Doppler ultrasound in differentiating benign from malignant endometrial disease is controversial, with significant overlap in the Doppler indices, though some reports have suggested the presence of low-impedance flow in endometrial carcinoma [46].