Key Facts
Gout
- •
Clinically significant attacks may occur many years before radiographic changes are evident.
- •
Radiographs classically show well-defined erosions with overhanging edges and sclerotic borders.
- •
Soft tissue nodules (tophi) may calcify or ossify.
- •
Classically, gout involves the first metatarsophalangeal joint.
- •
Lesions of gout are randomly distributed in the hand.
- •
Osteoporosis is not a feature of gout.
- •
Gout occurs concomitantly with calcium pyrophosphate dihydrate deposition (CPPD) in 40% of patients.
CPPD Disease
- •
CPPD disease may be a secondary finding in patients with gout, primary hyperparathyroidism, or hemochromatosis.
- •
CPPD disease may mimic gout (then termed pseudogout ), infection, or neuropathic arthropathy.
- •
Calcification of hyaline and fibrocartilage structures (chondrocalcinosis) is a hallmark of the disorder although not pathognomonic.
- •
The arthropathy of CPPD disease commonly involves the second and third metacarpophalangeal, radiocarpal, and patellofemoral joints.
- •
CPPD disease causes “drooping” osteophytes and prominent subchondral cysts.
Hydroxyapatite Deposition Disease (HADD)
- •
HADD may be acutely symptomatic and cause fever, increased C-reactive protein, and increased erythrocyte sedimentation rate.
- •
Crystals are beyond the resolution of light microscopy, making pathologic diagnosis cumbersome in routine clinical work.
- •
HADD typically appears on radiographs as amorphous globular calcifications in and around joints.
- •
HADD most commonly involves the shoulder.
- •
HADD may mimic malignancy when it causes erosion of adjacent bone and bone marrow edema.
Several types of crystals may deposit in joints. Crystal deposition may lead to symptoms, such as those due to inflammation or may be asymptomatic.
Crystal deposition may be symptomatic or asymptomatic.
Gout, calcium pyrophosphate dihydrate deposition (CPPD) disease, and calcium hydroxyapatite deposition disease (HADD) are common examples. Deposition of these respective crystals in and around joints will produce changes typical of each disease.
GOUT
Writing about gout in 1683, Thomas Sydenham captured the baffling nature of the disease at that time: “Either men will think that the nature of gout is wholly mysterious and incomprehensible, or that a man like myself, who had suffered from it thirty-four years, must be of a slow and sluggish disposition not to have discovered something respecting the nature and treatment of a disease so peculiarly his own.”
Gout has been known since antiquity. Early descriptions deemed it an affliction of wealthy adult men, but its cause (an extracellular urate supersaturation that results in the deposition of monosodium urate crystals [MSU] in the tissues) was not identified until the last half of the 20th century.
Extracellular urate supersaturation leads to monosodium urate crystal deposition.
Crystal deposition incites a complex inflammatory response that damages the tissue. The accumulation of these crystals may be idiopathic, a result of enzyme deficiencies, or the consequence of a myriad of disease states (e.g., renal disease, hyperparathyroidism, hypoparathyroidism, myeloproliferative disorders, diuretic use). A diet rich in red meat, seafood, and liquor also increases the incidence of gout. Saturnine gout is caused by chronic ingestion of homemade liquor (i.e., moonshine) contaminated with lead ( Table 26-1 ).
| Cause | Frequency | Examples |
|---|---|---|
| Uric acid overproduction | 10% | Primary: Idiopathic, Lesch-Nyhan syndrome (HGPRT deficiency) Secondary: Increased cell turnover (hematologic malignancies, psoriasis, Paget’s disease, chemotherapy), increased purine intake (alcohol), accelerated ATP degradation (alcohol, muscle overexertion) |
| Uric acid underexcretion | 90% | Primary: Idiopathic Secondary: Renal insufficiency, inhibition of tubular urate secretion (ketoacidosis and lactic acidosis), enhanced tubular reabsorption (diuretics, and dehydration), drugs (cyclosporine, pyrazinamide, ethambutol, low-dose ASA), lead, alcohol |
Clinically, gout may take the form of intermittent acute attacks of a red, swollen, painful joint or chronic arthropathy. White men who are middle aged to elderly are most commonly affected. Gout is uncommon in children and teenagers but may affect some young persons.
Gout is very uncommon in premenopausal women and is common in transplant patients.
Diagnosis is based on the presence of hyperuricemia (although the concentration of serum uric acid may be within normal limits), clinical history, radiographic findings, MSU crystal identification, or rapid symptom resolution after colchicine therapy.
Most individuals with hyperuricemia do not develop clinical gout.
MSU forms needle-shaped crystals that exhibit strong negative birefringence under polarized light microscopy ( Figure 26-1 ). When oriented parallel to the compensator, the crystals appear yellow, whereas they appear blue when oriented perpendicularly.
MSU crystals are negatively birefringent.

The crystals may be intracellular (e.g., within neutrophils) or extracellular. A secondary finding is an elevated white blood cell count in synovial fluid caused by the crystal-induced inflammation.
Osteoarticular Imaging Features
Radiographs
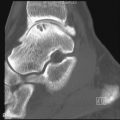
Radiographic findings of gout are pathognomonic ( Figure 26-2 ) ( Box 26-1 ). Round to oval intraarticular or paraarticular erosions have well-defined sclerotic borders, giving them a punched-out appearance. The bone adjacent to the erosions may extend outward to produce an “overhanging margin.”
Erosions classically have sclerotic bases and new bone formation (“the overhanging margin of gout”).

Normal or near-normal bone density
Normal alignment
Asymmetric joint involvement
Soft tissue masses that may calcify or ossify
Cartilage spaces may remain normal
Intraarticular and extraarticular erosions
Erosions have sclerotic bases and “overhanging margins”
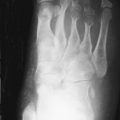
Additional productive changes include enlargement of the phalangeal bases. Bone density in gout is normal unless disuse osteoporosis supervenes. The joint spaces are often well preserved until late in the disease, and the relative lack of cartilage destruction may help differentiate gout from other erosive arthropathies. Soft tissue tophi may be of homogeneous increased density or may contain focal calcification, which is more common in patients with altered calcium metabolism. The tophi cause a nodular appearance ( Figure 26-3 ). They are more common in patients who have had gout for many years or who have responded poorly to treatment.

Gout is asymmetric and polyarticular. Unfortunately, these typical radiographic changes are not visible until about 10 years after clinical presentation and early diagnosis is largely clinical. Advanced changes of gout ( Figure 26-4 ) are seldom observed now because of improved diagnosis and treatment.

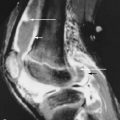
Gout most commonly affects the feet. In more than half of patients, the initial sign is acute inflammation of the first metatarsophalangeal (MTP) joint. The other MTP joints, interphalangeal joints, midfoot, and hindfoot may also be involved. In the hand, the distal interphalangeal, proximal interphalangeal, and intercarpal joints are often involved. The bones may enlarge because of reactive new bone formation ( Figure 26-5 ). When this occurs in the phalangeal bases or the ulnar styloid process, it produces a mushroom appearance. Within the elbow, the olecranon may be eroded with tophus formation in the overlying bursa ( Figure 26-6 ).


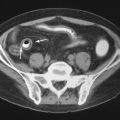
Large joints, such as the knee, are less commonly affected than are the small joints of the feet and hands. Within the knee, marginal erosions ( Figure 26-7 ) and involvement of the prepatellar bursa are typical. Within the pelvis, the sacroiliac joints may be markedly eroded ( Figure 26-8 ). Spinal involvement is seldom observed. However, gout in the spine has been reported in vertebral bodies, intervertebral disks, posterior elements, and facets. Because gout in the spine is so rare, infection and neoplasm are often diagnostic alternatives.


Focal collections of crystals (i.e., tophi) occur in the synovium, ligaments, tendons, and bursae. When tophi are intraosseous, they have a predilection for the patella and almost never calcify, in contrast to the soft tissue tophi that sometimes contain calcifications.
Lytic lesions of the patella are usually benign.
Intraosseous tophi may have an aggressive appearance simulating that of a malignancy. Extraarticular tophi tend to occur in the olecranon bursa, the prepatellar bursa, and the dorsum of the foot. Pressure from tophi can produce extraarticular erosions and mass effect on adjacent neurovascular structures, resulting in carpal tunnel syndrome or paraplegia. Erosion of bone and soft tissues by tophi sometimes leads to pathologic fracture or tendon rupture.
Conditions associated with nontraumatic tendon rupture include gout, systemic lupus erythematosus, rheumatoid arthritis, obesity, Ciprofloxacin use, and diabetes.
Urate crystals can also penetrate the cartilage and extend into the medullary canal, producing small, circular, calcified deposits in the bone. Punctate intraosseous calcifications that collect in the subchondral bone can mimic bone infarcts and enchondromas.
Gout sometimes coexists with other articular disorders (e.g., osteoarthritis, CPPD disease). When radiographic findings of multiple entities occur together, diagnosis may be difficult, especially when gout coexists with infection.
Computed Tomography
Erosions and soft tissue tophi show up clearly on computed tomography (CT) ( Figure 26-9 ). MSU crystals have a characteristic density of 160 Hounsfield units (HU). This measurement is substantially lower than that of calcium, which is 450 HU. CT can be especially helpful in defining abnormalities in areas such as the spine that have multiple overlapping structures on radiographs.

Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is helpful in evaluating the extent of gout, especially in the spine, but it has limited diagnostic usefulness.
MRI has limited diagnostic value in patients with gout but demonstrates the degree of bone and soft tissue involvement better than radiographic or clinical examination.
A tophus may have low to intermediate signal on T1-weighted images and a variable low to high signal on T2-weighted images ( Figure 26-10 ).
Tophi demonstrate intermediate signal on T1-weighted images and variable signal on T2-weighted images.

Tophi may show homogeneous, inhomogeneous, or only peripheral enhancement ( Figure 26-11 ). Some tophi may contain focal fluid collections. The varied appearance of gout on MRI may be related to the amount of calcium within a tophus. MRI is useful, however, for identifying tophi that are not clinically evident.

Scintigraphy
In active gout, radionuclide bone scanning shows nonspecific increased radiotracer uptake. Neoplasm, trauma, and infection can have a similar appearance. Gout can mimic infection clinically and radiographically. Unfortunately, even three-phase bone scanning, which usually has a high specificity for infection, may produce false-positive findings because of gout. Tophi are hypermetabolic on positron emission tomography (PET). A case report using PET imaging of an intraosseous patellar tophus identified metabolic activity that was less than that of malignancy, which suggests a possible role for PET in differentiating gout from neoplasm. Indium 111-labeled leukocyte imaging can sometimes help differentiate gout from infection, but there have been false-positive cases. Cases of infection typically show increased uptake on indium-labeled white blood cells scans. Increased radiotracer uptake is seen in inflamed joints including those with crystal disease when imaged using Tc-99m ciprofloxacin. This technique has been proposed as a method for monitoring response to treatment. Cases that are healing typically exhibit decreasing uptake.
Arthrography
Conventional arthrography has little use in the diagnosis or follow-up of gout. Fluoroscopy can guide joint aspiration for crystal analysis, and the injection of contrast material may ensure accurate intraarticular needle placement.
Ultrasonography
Ultrasound is useful for guiding joint aspirations and biopsy. It can also be used to identify joint effusions and alterations of the surrounding soft tissue structures, such as tendons and ligaments. On Doppler ultrasound, tophi have a nonspecific heterogeneous appearance with a hypervascular rim. After tophi are identified, they are easy to measure with ultrasound, which can be useful for follow-up. Ultrasound is also helpful for assessing renal complications of hyperuricemia.
Extraarticular Imaging Features
Gouty tophi may occur in many soft-tissue sites, including cartilaginous involvement of the nose and the helix of the ear. Hyperuricemia may also affect the kidneys, causing nephrolithiasis and chronic urate nephropathy. Urate calculi are typically opaque on noncontrast CT examinations. Tophi in the corpus cavernosum may cause erectile dysfunction. Reflex sympathetic dystrophy has been reported as a complication of gout. One case report described a gouty tophus that tracked alongside the iliopsoas muscle and mimicked an intrapelvic abscess.
Algorithms and Recommendations
In cases suspected of gout, survey radiographs should include anteroposterior (AP), oblique, and lateral views of the feet and a posteroanterior (PA) view of the hands. Symptomatic areas should also be imaged with radiographs. When gout is suspected but not clearly defined, CT or MRI may be of use, especially for imaging the spine.
In acute monarthritis, radiographs should be considered prior to arthrocentesis to assess for fracture, especially if there is a history of trauma or risk factors for osteoporosis.
CPPD DISEASE
In CPPD disease, crystal deposition is usually idiopathic but also may be hereditary or associated with several underlying diseases.
Some causes of CPPD disease include familial, hyperparathyroidism, hemochromatosis, ochronosis, or hypophosphatasia.
The two familial forms of CPPD disease are caused by alterations on the long arm of chromosome 8 or on the short arm of chromosome 5. CPPD is associated with metabolic diseases, such as hyperparathyroidism, hemochromatosis, ochronosis, or hypophosphatasia. In cases with intermittent acute attacks of joint pain mimicking the clinical presentation of gout, CPPD disease is referred to as pseudogout .
Clinical manifestations of CPPD disease include asymptomatic, pseudorheumatoid, pseudogout, pseudoneuropathic, pseudoosteoarthritis.
Although cartilage calcification (chondrocalcinosis) is most commonly associated with CPPD deposition disease, other crystals (e.g., dicalcium phosphate dihydrate or calcium hydroxyapatite) may precipitate in cartilage to produce chondrocalcinosis. Calcium pyrophosphate dihydrate crystals are rod shaped or rhomboid and, on polarized light microscopy, have positive birefringence (blue when parallel to the compensator axis).
CPPD crystals are smaller and less brightly refringent on polarized light microscopy compared with MSU crystals and may, therefore, often missed.
Osteoarticular Imaging Features
Radiographs
Radiographs are the most important imaging tests in the evaluation of CPPD disease. The hallmark finding is chondrocalcinosis of hyaline cartilage or fibrocartilage ( Figure 26-12 ). Chondrocalcinosis of fibrocartilage is most commonly observed in the knee menisci, wrist triangular fibrocartilage complex, symphysis pubis ( Figure 26-13 ), and acetabular labrum.
Essentially all patients with chondrocalcinosis may be discovered if radiographs of three sites: the knees, wrists, and pelvis (pubic symphysis) are obtained.


Crystals may also collect in tendons, ligaments, synovium, bursae, and joint capsules. Chondrocalcinosis in hyaline cartilage parallels the underlying bone and appears as thin linear deposits ( Figure 26-14 ). Crystal deposition in cartilage causes accelerated cartilage breakdown and a radiographic appearance that mimics degenerative joint disease. Changes may be so severe that they mimic neuropathic or Charcot-like destruction of the joint. Joint changes are typically bilateral and symmetric.

The key differentiating factor between CPPD disease and degenerative joint disease is the location of the joint changes.
CPPD disease typically affects the radiocarpal, patellofemoral, and second and third MCP joints.
CPPD disease tends to affect areas not typically involved by degenerative joint disease (e.g., the radiocarpal, patellofemoral, second and third MCP joints, and the shoulder and elbow). The lack of erosion differentiates CPPD disease from gout and rheumatoid arthritis. The lack of osteopenia differentiates CPPD disease from septic arthritis and rheumatoid arthritis.
The most commonly affected joint is the knee, where cartilage space narrowing and osteophytes may involve all three compartments. Severe changes in the patellofemoral joint are suggestive of CPPD disease ( Figure 26-15 ), particularly when found in isolation.
Isolated patellofemoral cartilage space narrowing suggests CPPD arthropathy.