Fig. 2.1
A morbidly obese, 55-year-old woman with endometrial cancer was found to have periportal lymphadenopathy. Diagnostic contrast-enhanced CT image of the abdomen (a) shows a 1.5 cm lymph node (arrowhead) immediately anterior to the common hepatic artery. Axial CT fluoroscopy image of the abdomen (b) shows a transhepatic approach to the lymph node (arrowhead). The core biopsy needle is centered within the lesion, anterior to common hepatic artery (arrows)
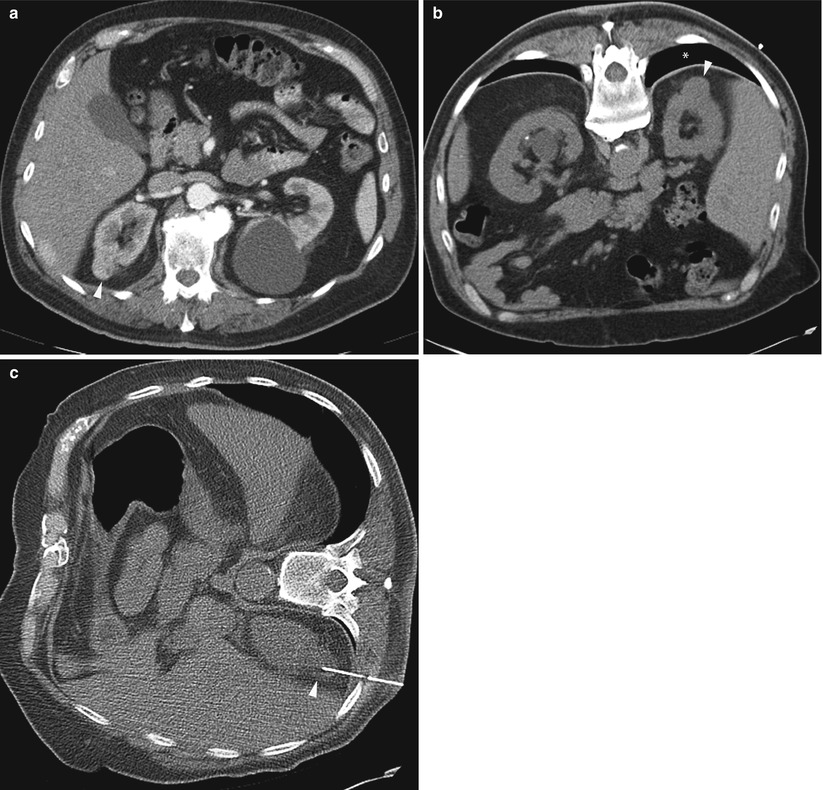
Fig. 2.2
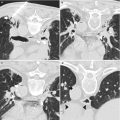
A 78-year-old man was referred for biopsy of a right kidney mass for consideration of percutaneous thermal ablation. Axial CT image of the abdomen (a) shows a solid enhancing mass (arrowhead) in the upper pole of the right kidney. Axial image of the abdomen in prone position (b) shows intervening lung (asterisk). When patient was positioned in right lateral decubitus (c), the lung volume decreased and a safe window appeared for biopsy of the renal mass (arrowhead). A pathologic assessment revealed renal cell carcinoma, clear cell type, nuclear grade 3
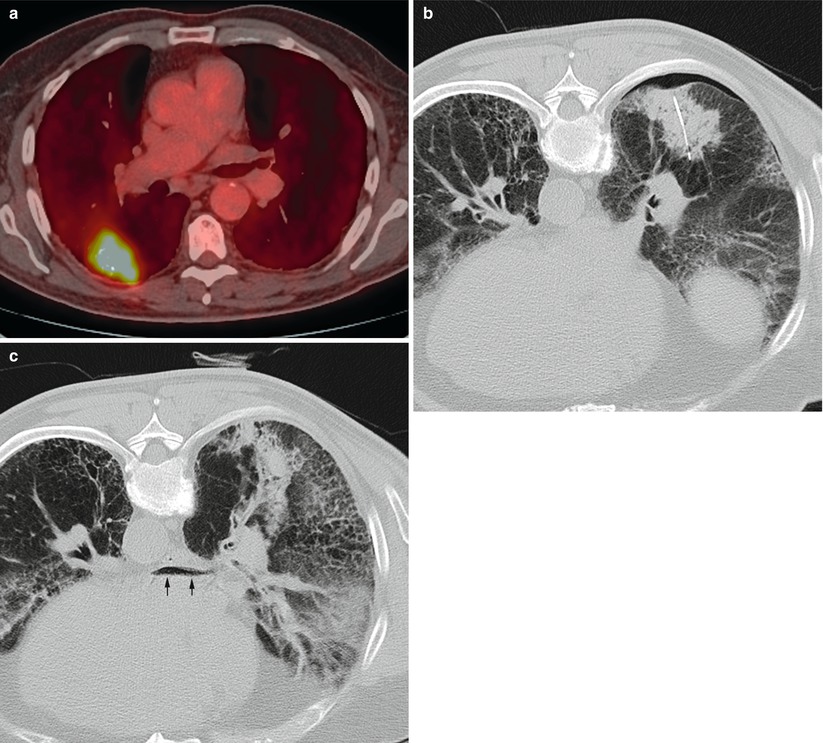
Fig. 2.3
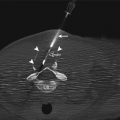
A 75-year-old man with severe emphysema was found to have a mass in the right lower lobe. Axial PET/CT image of the mass (a) shows high metabolic activity. CT image of the patient in prone position shows the needle tip within the mass (b) and small pneumothorax. After aspiration of the pneumothorax, axial CT image of the chest (c) shows air within the left atrium (arrows). Systemic air embolism is best treated with hyperbaric oxygen
Disadvantages
Density differences between normal tissues and tumors may not be sufficiently large to allow tumor pathology to be distinguished from normal tissue on noncontrast CT images (Fig. 2.4). While contrast-enhanced CT can be performed to help guide the biopsy procedure (Fig. 2.5) [4], contrast enhancement is often transient and may not last for the entire procedure.
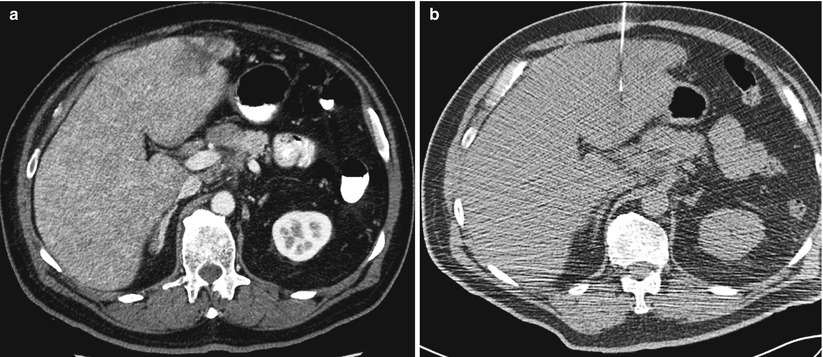
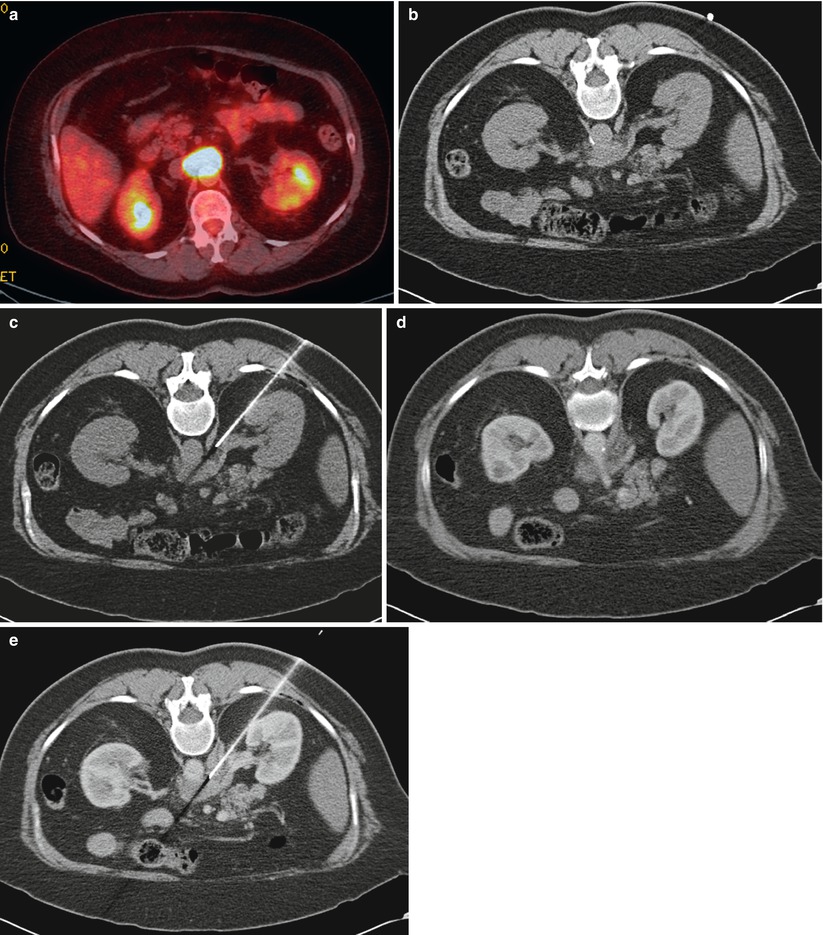

Fig. 2.4
A 65-year-old man with history of Merkel cell carcinoma was referred for liver biopsy. Diagnostic axial CT image of the abdomen after administration of contrast (a) shows a lesion in segment II of the liver. A noncontrast axial CT image of the abdomen (b) at the time of biopsy did not clearly show the tumor. The lesion was targeted by anatomic landmarks. Immediate assessment of the FNA samples by cytopathologist confirmed metastatic Merkel cell carcinoma. Also note that the diagnostic CT scan (a) was performed with the patients arms elevated above his head. Whereas his arms were left on his side during CT-guided biopsy (b). The artifact from the arms can hinder visualization of subtle lesions

Fig. 2.5
A 62-year-old woman with lymphoma underwent restating PET/CT examination. Axial fused PET/CT image of the abdomen (a) shows a metabolically active soft tissue mass encasing the celiac axis. Noncontrast axial CT image of the abdomen in prone position (b) shows the soft tissue mass abutting the anterior wall of aorta. A guide needle was advanced to the edge of the lesion (c). Immediately before insertion of the core biopsy needle, contrast-enhanced CT images were obtained demonstrating the celiac artery (d). The guide needle is identified approximately 10 mm inferior to the celiac artery (e). Contrast-enhanced axial CT image of the lesion (e) confirms the absence of any major vessels in the path of the needle
Another disadvantage of CT guidance is that CT imaging is limited to an axial plane. As such, the biopsy planning route is often severely restricted. A multidetector CT with automated reconstruction of images in arbitrary planes alleviates some of these limitations (Fig. 2.6).
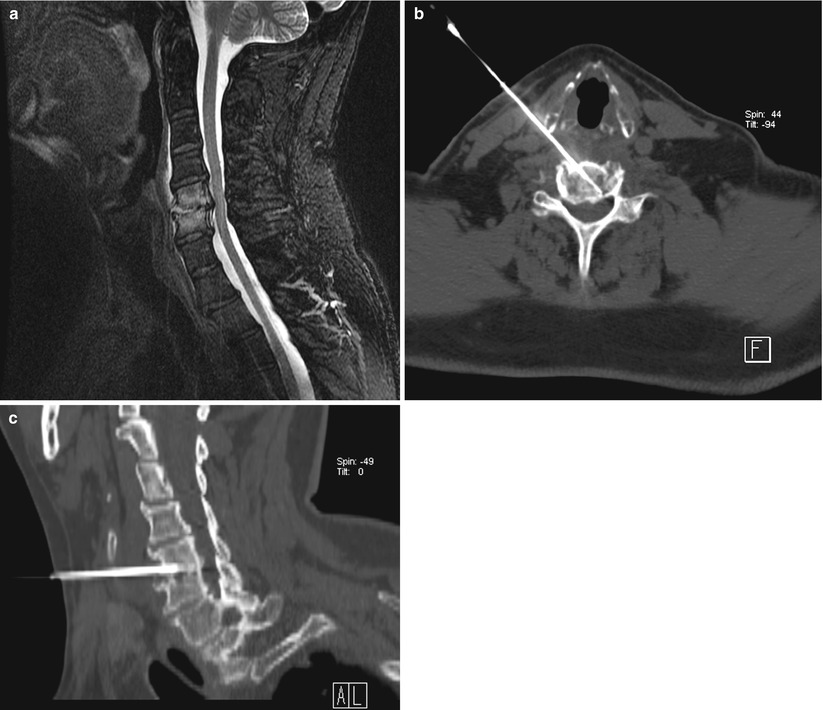

Fig. 2.6
A 53-year-old man with leukemia was referred for biopsy of cervical spine. Sagittal T2 MRI of the cervical spine (a) shows high signal intensity in C5 and C6 vertebral bodies with involvement of the disc space. Axial-oblique (b) and sagittal-oblique (c) multiplanar reformatted images show the position of the needle within the area of interest
Another major disadvantage of conventional CT for image-guided biopsies is the lack of real-time imaging. Although CTF provides near-real-time imaging, the radiation dose to the patient and operator may prohibit its routine, unrestricted utilization [5–8]. Several studies have documented higher radiation exposure to patients and radiologists from both direct skin exposure and scattered radiation during CTF-guided procedures than during conventional, sequential CT-guided procedures [2, 5–10].
Applications
Although CT can be used to biopsy masses in virtually any part of the body, CT-guided biopsy is most often used for sampling small abdominal (Fig. 2.1), retroperitoneal (Fig. 2.7), thoracic (Fig. 2.8), and musculoskeletal (Fig. 2.9) lesions that are not well visualized on ultrasonography or fluoroscopy [3]. CTF-guided biopsy has been most commonly used for sampling pulmonary nodules. CTF, with its near-real-time imaging capabilities, allows the interventional radiologist to time the needle puncture with the patient’s breathing, which helps in targeting a moving nodule and avoiding ribs during thoracic biopsies. Most studies on the use of CTF-guided biopsy of pulmonary lesions have shown high rates of technical success [11–16]. CTF-guided transthoracic biopsy procedures are associated with shorter procedural times and fewer needle punctures than are conventional CT-guided biopsies [8, 9, 11–17].
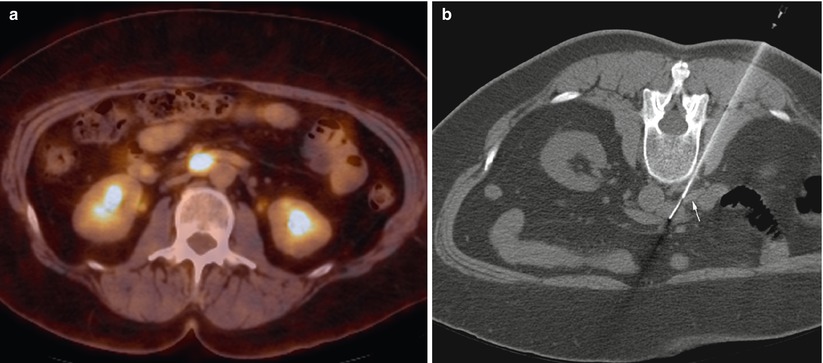
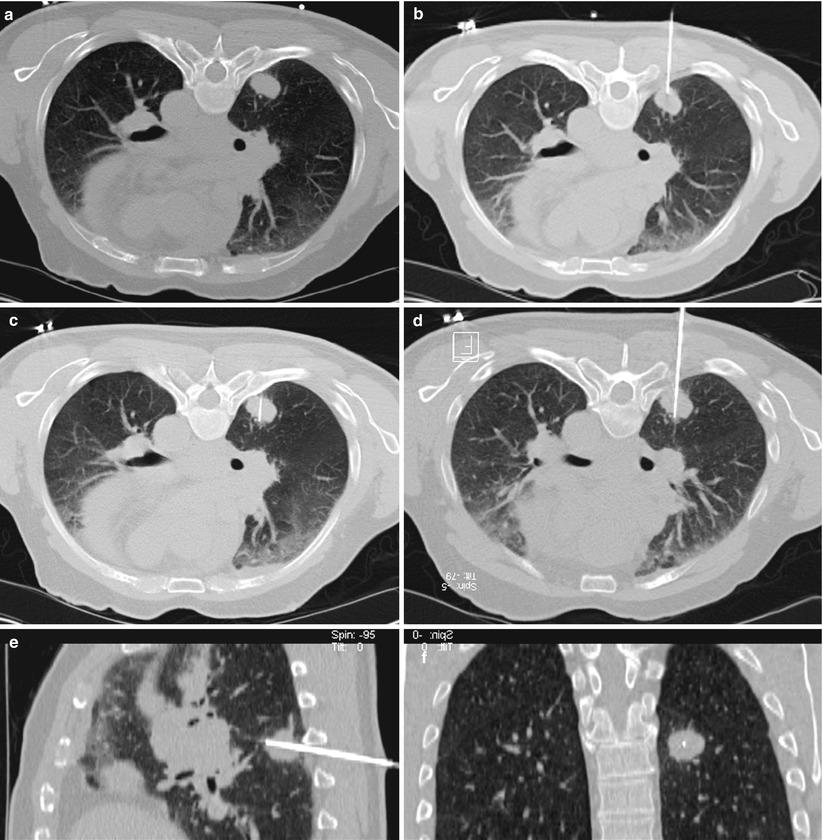
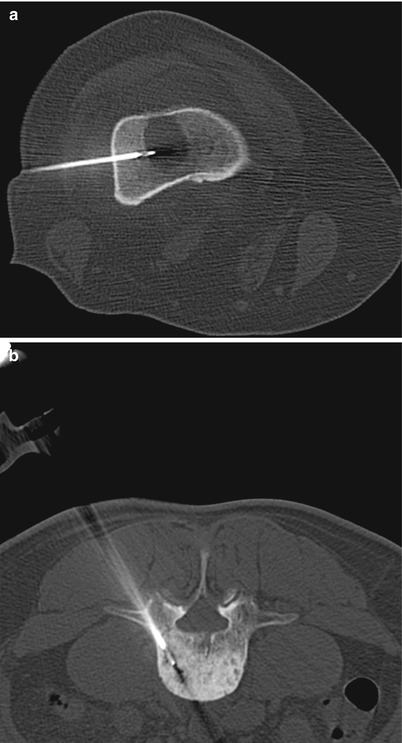

Fig. 2.7
A 56-year-old woman with history of ovarian cancer underwent PET/CT for restaging. Axial PET/CT image of the abdomen (a) shows a mass with high metabolic activity in aortocaval space. Axial CT image of the abdomen in prone position (b) shows the needle entering the mass from a posterior approach. The white arrow shows the inferior vena cava

Fig. 2.8
A 57-year-old man with newly diagnosed right lung mass was referred for biopsy. Axial CT image of the chest in prone position (a) shows the right lung mass. Overlying rib and costovertebral joint limit access to the lesion in a true axial plane. The gantry was tilted 10° caudo-cranial. Axial-oblique CT fluoroscopy image of the chest (b) demonstrates entire length of the needle extending to the posterior border of the tumor. True axial CT image of the chest (c) shows only the tip of the needle within the lesion. Multiplanar reformatted images demonstrate entire length of the needle in axial-oblique (d) and sagittal-oblique (e) planes. A coronal reconstructed image of the patient in prone position (f) shows the tip of the needle within the center of the mass

Fig. 2.9
Both lytic (a) and sclerotic (b) bone lesions can be visualized and targeted for biopsy. Biopsy of the lytic lesion in the distal right femur (a) showed metastatic cervical cancer. Biopsy of a sclerotic lumbar vertebral body tumor (b) showed metastatic neuroendocrine carcinoma
In addition, the needle tip and the biopsy target can be visualized during sampling to ensure that the needle tip is indeed within the target. CTF also facilitates placement of the biopsy needle at the edge of a lesion that has a predominantly necrotic center. With CTF, complications are recognized immediately, allowing for rapid institution of appropriate therapy.
Although CTF is not routinely used for abdominal and pelvic biopsies, CTF guidance can be useful in certain circumstances [18, 19]. CTF can be used to guide biopsy of liver lesions that show transient enhancement after intravenous injection of contrast medium, masses with difficult or narrow access routes, and masses close to the diaphragm or critical vascular structures [20, 21]. CTF can assist in needle placement in omental or mesenteric lesions that move with respiration or that may be intermittently surrounded by bowel loops.
Techniques
Setup
For CT-guided biopsies, a sterile field is set up on a movable procedure cart in the CT room. The interventional radiologist reviews existing images, and then the patient is placed in a position that facilitates needle insertion in a safe and efficient manner from the skin surface to the target. Patients can be placed in supine, prone, lateral, or lateral oblique positions as needed, but it is crucial to select a stable and comfortable position for the patient. The patient should remain in the same position after the initial planning images are obtained. Streak artifacts caused by the patient’s arms can be reduced by positioning the arms outside the imaging field (Fig. 2.4). Space within the gantry can be maximized by placing the table at the lowest position possible.
A radiopaque marker or grid is placed over the intended area of puncture. Radiopaque grids are commercially available; however, grids can be created on site using various types of catheters that are cut to a length of 10–20 cm, placed parallel at 1- to 2-cm intervals, and taped at the ends. The simplest radiopaque marker consists of a single piece of angiographic or drainage catheter placed on the patient in longitudinal fashion. Alternatively, one can use the laser lights that are built in the gantry together with the grid which can be displayed on the patient’s image to determine the needle puncture site.
For most interventions, a spiral scan of the area of interest is performed, including the marker or grid. In most cases, intravenous administration of contrast medium is unnecessary, but occasionally noncontrast images do not allow differentiation of the target from adjacent vascular structures. Tumors within solid organs may be isodense with their surrounding tissue on noncontrast images, in which case the contrast medium may be given intravenously to delineate the vascular structures or to differentiate the lesion from normal parenchyma (Fig. 2.5) [4].
The interventional radiologist identifies the target and then determines a path for placement of the biopsy needle or device. The needle path depends on the organ of interest and the intervening anatomy. In general, the path should be kept short, but it should also avoid certain anatomic structures such as bone, vessels, bowel, or other organs to render the procedure as safe as possible. Once the needle insertion site is determined, the patient is placed into the gantry. The positioning laser light and the radiopaque marker together provide adequate information to localize the exact site for needle insertion. A small ruler and a skin marker are often necessary to mark the puncture site accurately on the skin. The skin is then prepared in a sterile manner, and a keyhole drape is placed over the puncture site. A sterile cover may be applied to the table-side control panel so that the radiologist can operate the CT scanner during the procedure.
Biopsy Technique
Most biopsies are performed under moderate sedation or local anesthesia. The skin entry site is infiltrated with local anesthetic agent (e.g., lidocaine 1 %), and sufficient local anesthetic agent should be injected at the interface of the pleura, peritoneum, or periosteum. A small stab incision is made to help the needle pass through the skin and subcutaneous tissues. CT guidance provides intermittent imaging, and thus the needle is inserted incrementally (1 to a few centimeters at a time) during CT-guided biopsy, and images are obtained after each manipulation to confirm proper advancement of the needle along the desired path.
CTF guidance provides continuous near-real-time imaging that allows monitoring of the needle insertion during CTF-guided biopsy. To avoid excessive radiation to the operator’s hand, needle-holding devices may be used [22]. Even with the use of needle-holding devices, radiation exposure to the operator and patient may not warrant real-time continuous-mode CTF. Alternatively, intermittent CTF can be performed after incremental advancement of the needle [23].
The operator must be cognizant of two angles when performing CT- or CTF-guided biopsy. Most CT-guided biopsies are performed in a true axial plane. This approach provides the easiest imaging protocol to confirm needle placement. Therefore, the operator has to keep the needle in one axial plane perpendicular to the Z axis. Cranial or caudal angulation of the needle can be detected on sequential CT images, and the path of the needle can be adjusted accordingly. One can take advantage of the laser light of the scanner for alignment of the needle in an axial plane. When the laser light shines on the skin entry site and the hub of the needle, it confirms the positioning of the needle in the imaging plane. The simplest CT-guided biopsy plan is one in which the needle is inserted perpendicular to the skin. However, this approach is not always feasible, depending on the intervening organs or structures that may have to be avoided. Therefore, the second angle that the operator should observe is the steepness of the approach with respect to the skin’s surface. Placement of the needle can be guided with the naked eye; however, this is more easily accomplished if the patient is taken out of the gantry and the puncture site is placed directly in front of the operator to avoid issues related to parallax.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree