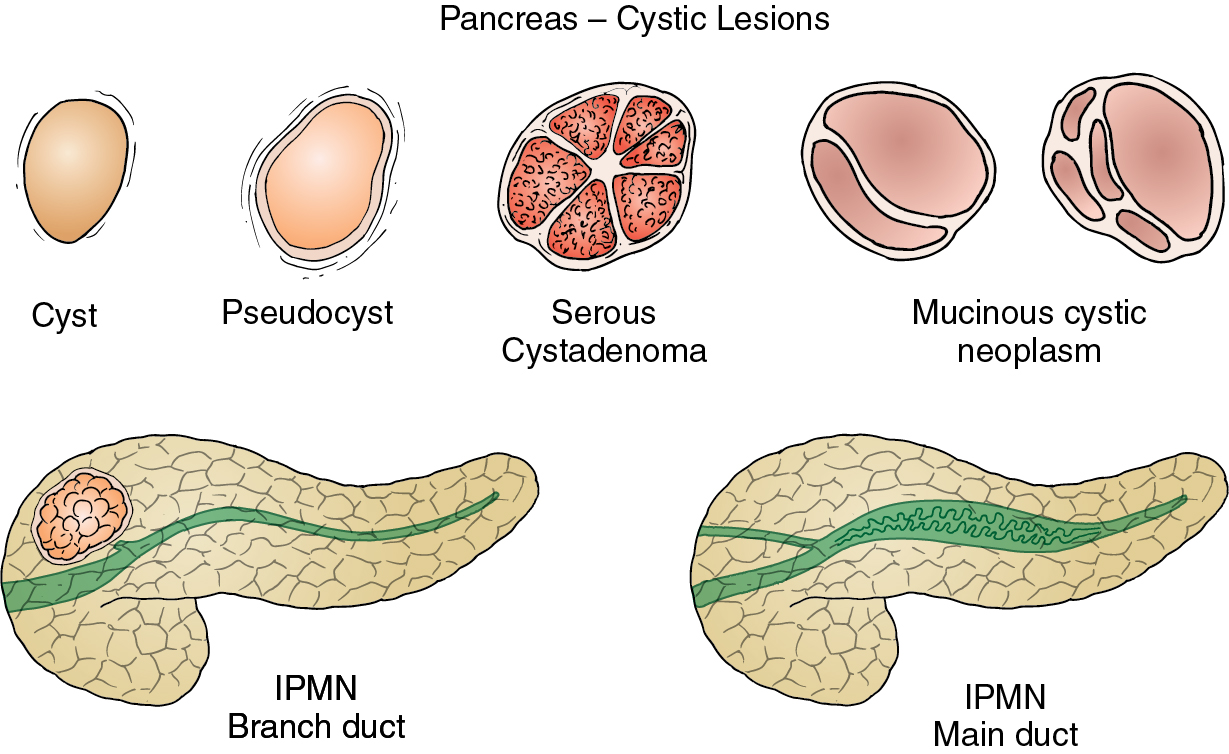
Anatomy, embryology, pathophysiology ( fig. 14.1 )
- ◼
Serous cystadenoma: inactivation of von Hippel-Lindau ( VHL ) gene.
- ◼
Mucinous cystic neoplasms (MCNs): closeness of left primordial gonad to the dorsal pancreatic bud during development, with possible incorporation of ovarian stroma into developing pancreatic bud, has been suggested to play a role in the cause of MCNs. This observation could explain the female occurrence and predilection for the tail and body of the pancreas.
- ◼
Intraductal papillary mucinous neoplasms (IPMNs): different genetic mutations have been reported in IPMNs, including inactivation of tumor suppressor genes, such as TP53 , and activation of oncogenes, such as KRAS . A multistep process is probably involved in the progression from hyperplasia to invasive carcinoma.
- ◼
Solid and pseudopapillary epithelial neoplasms (SPENs): mutations in exon 3 of the beta-catenin gene have been found in almost all SPENs.
- ◼
Cystic pancreatic neuroendocrine tumors (NETs): many chromosomal losses have been reported; some of them are associated with more aggressive biological behavior. Usually larger NETs harbor more genetic alterations than smaller lesions.
- ◼
Pseudocyst: walled-off collection of pancreatic secretions after disruption of the duct because of pancreatitis.
Techniques
Computed tomography
- ◼
Superior resolution helps in detection of cystic lesions and morphologic characterization including size, presence of calcifications, septations, central scar, wall thickness, and evaluation for solid enhancement (i.e., mural nodule).
- ◼
Evaluate pancreatic duct dilation ( Fig. 14.2 ), stricture, or any communication with cyst.
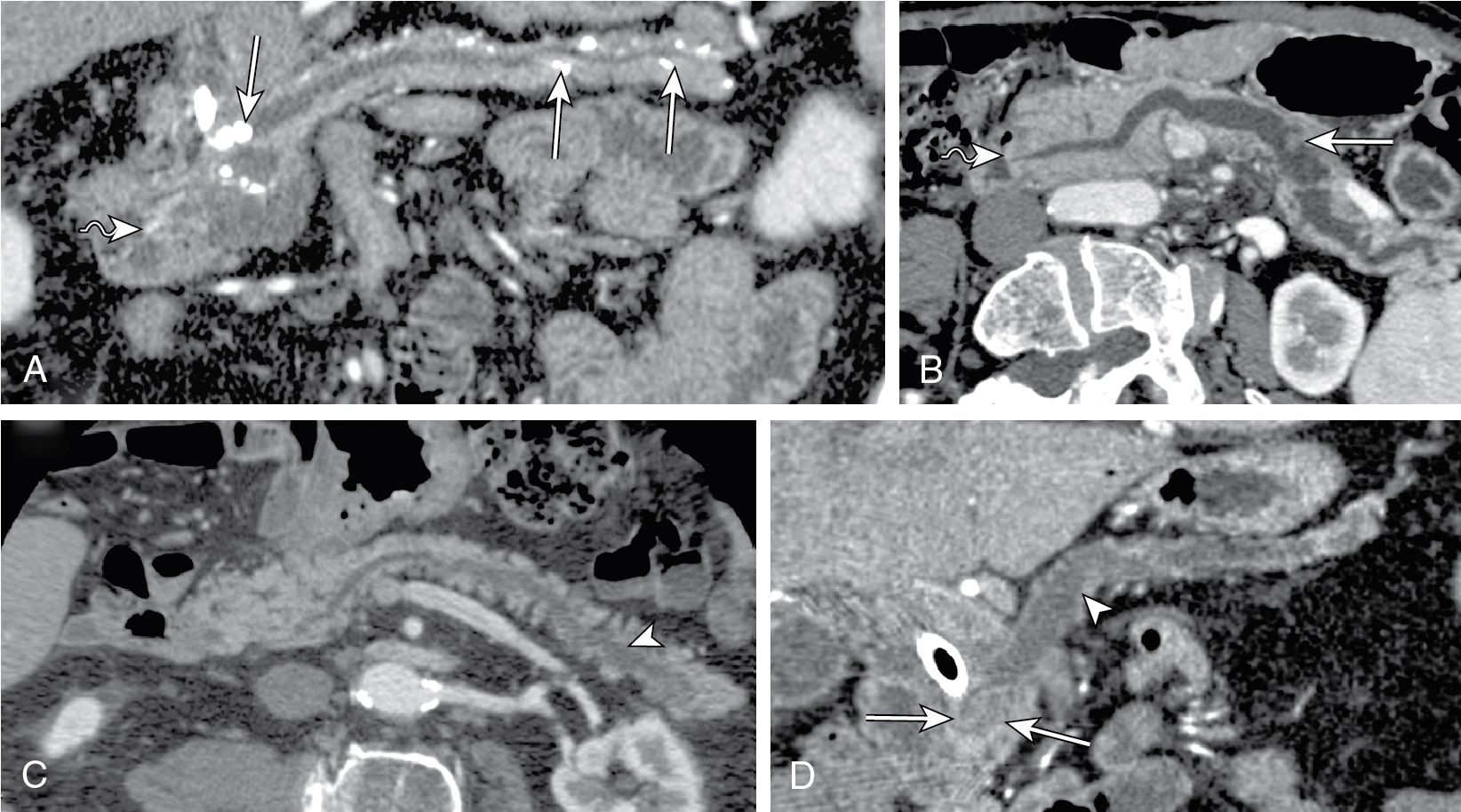
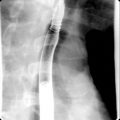
Fig. 14.2
Multiple reformatted computed tomography images depicting the differential diagnosis for pancreatic duct (PD) dilatation. A, In chronic pancreatitis PD, dilatation is associated with parenchymal atrophy out of proportion to ductal dilatation, ductal calculi ( arrows ), and lack of papillary bulge ( wavy arrow ). In intraductal papillary mucinous neoplasm (IPMN), either diffuse (B) or segmental IPMN (C) PD dilatation manifests as proportional parenchymal atrophy without abrupt PD narrowing. Also in diffuse form, bulging papilla ( wavy arrow , B) is seen. D, In adenocarcinoma, PD manifests as abrupt change in ductal diameter with a focal stenosis ( arrowhead ) and obstructing mass ( arrows ).
(From Sahani DV, Samir AE. Abdominal Imaging , ed 2. Philadelphia: Elsevier; 2017.)
- ◼
Categorization of patients into surgical and nonsurgical groups.
- ◼
Follow-up in patients in whom surgery is not indicated initially.
- ◼
For postoperative management and follow-up.
Magnetic resonance imaging/magnetic resonance cholangiopancreatography
- ◼
Enables better cyst characterization than computed tomography (CT) because of superior soft tissue resolution ( Fig. 14.3 ).
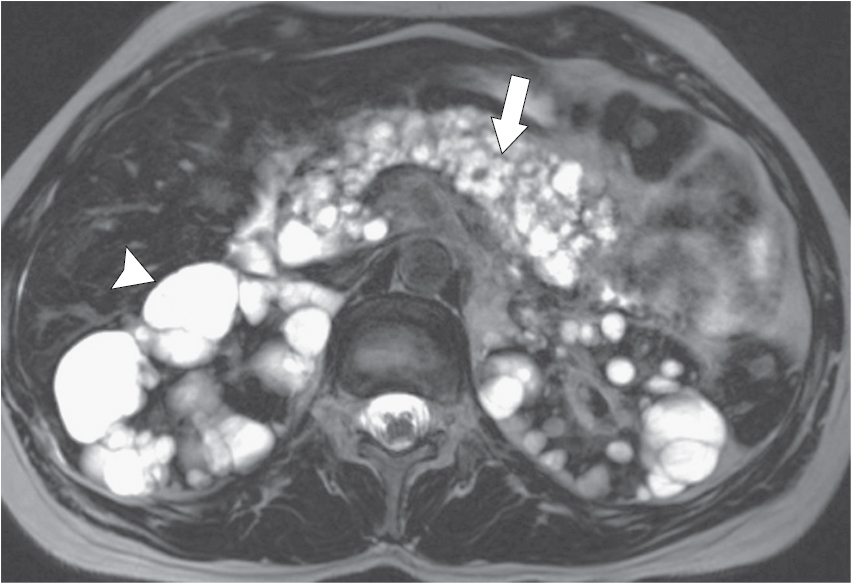
Fig. 14.3
Axial T2-weighted magnetic resonance imaging in a 32-year-old woman with von Hippel-Lindau disease. There are numerous simple pancreatic ( arrow ) and renal cysts ( arrowhead ).
(From Boland GW. Gastrointestinal Imaging: The Requisites , ed 4. Philadelphia: Saunders; 2014.)
- ◼
For pancreatic duct evaluation, to detect any mural nodules or septations.
- ◼
Follow-up in patients having higher radiation risk (e.g., those <50 years of age).
- ◼
In patients with contraindications to iodinated contrast agents (e.g., patients in renal failure).
- ◼
Identifies hemorrhagic complications within the cyst in different stages.
- ◼
Defines the communication with the pancreatic duct on magnetic resonance cholangiopancreatography (MRCP) images, thus establishing the diagnosis of side branch intraductal papillary mucinous neoplasm of the pancreas in the majority of cases ( Fig. 14.4 ).

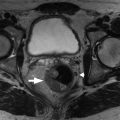
Fig. 14.4
Magnetic resonance cholangiopancreatography in a 74-year-old man demonstrating three small side branch intraductal papillary mucinous neoplasms ( arrows ).
(From Boland GW. Gastrointestinal Imaging: The Requisites , ed 4. Philadelphia: Saunders; 2014.)
Ultrasonography
- ◼
Poor performance in obese patients, operator dependent, comprehensive imaging difficult.
- ◼
Serous cystadenomas are composed of glycogen-rich serous fluid, which makes this tumor visible sonographically.
- ◼
Microcystic lesions may have a solid appearance.
- ◼
Contrast enhanced ultrasound improves characterization of serous cystadenomas with enhancement of septa and identification of microcystic features of the lesion.
Protocols
Computed tomography
- ◼
125 to 150 cc of iodinated contrast injected at a rate of 4 to 5 mL/s.
- ◼
Early arterial phase:
- ◼
20 seconds after contrast bolus.
- ◼
- ◼
Delayed arterial phase AKA pancreatic phase:
- ◼
40 to 50 second delay after bolus of iodinated contrast.
- ◼
Good for detection of tumor.
- ◼
- ◼
Portal venous phase:
- ◼
70 to 80 second delay.
- ◼
Look for hypodense liver metastases and for venous encasement.
- ◼
Magnetic resonance imaging
- ◼
T1-weighted gradient recalled echo (GRE) sequences with fat suppression.
- ◼
Axial noncontrast.
- ◼
Most sensitive for pancreatic lesions.
- ◼
- ◼
Axial arterial-capillary phase: 25 to 30 seconds delay.
- ◼
Axial pancreatic phase: 40 to 50 seconds delay.
- ◼
Axial portal vein phase: 70 to 80 seconds delay.
- ◼
Useful for portal vein evaluation, lymphadenopathy.
- ◼
- ◼
Coronal images: 5 to 10 minutes delay.
- ◼
Useful for cholangiocarcinoma, cholangitis, abscess, metastases.
- ◼
- ◼
T1-weighted GRE: in/out of phase.
- ◼
T2-weighted images.
Specific disease processes ( fig. 14.5 )
Serous cystadenoma
- ◼
Buzzword: “grandmother lesion”.
- ◼
Predominantly in women (75%), with mean age of 62 years.
- ◼
Some 30% to 39% of all pancreatic cystic neoplasms, slowly growing, benign lesions with low malignant potential.
- ◼
Incidentally discovered, unless mass effect on organs.
- ◼
Most in head (42%) or body/tail (48%), less often in the proximal body (7%), or diffusely through the pancreas (3%).
- ◼
Usually microcystic and, less commonly (10%), macrocystic or oligocystic.
- ◼
Classic microcystic serous cystadenomas have a “sponge-like” or “honeycomb-like” morphology, characterized by innumerable small cysts of a few millimeters in size. On imaging, fine external lobulations with central fibrous scar and/or stellate calcification; delayed enhancement of septations is characteristic ( Fig. 14.6 ).
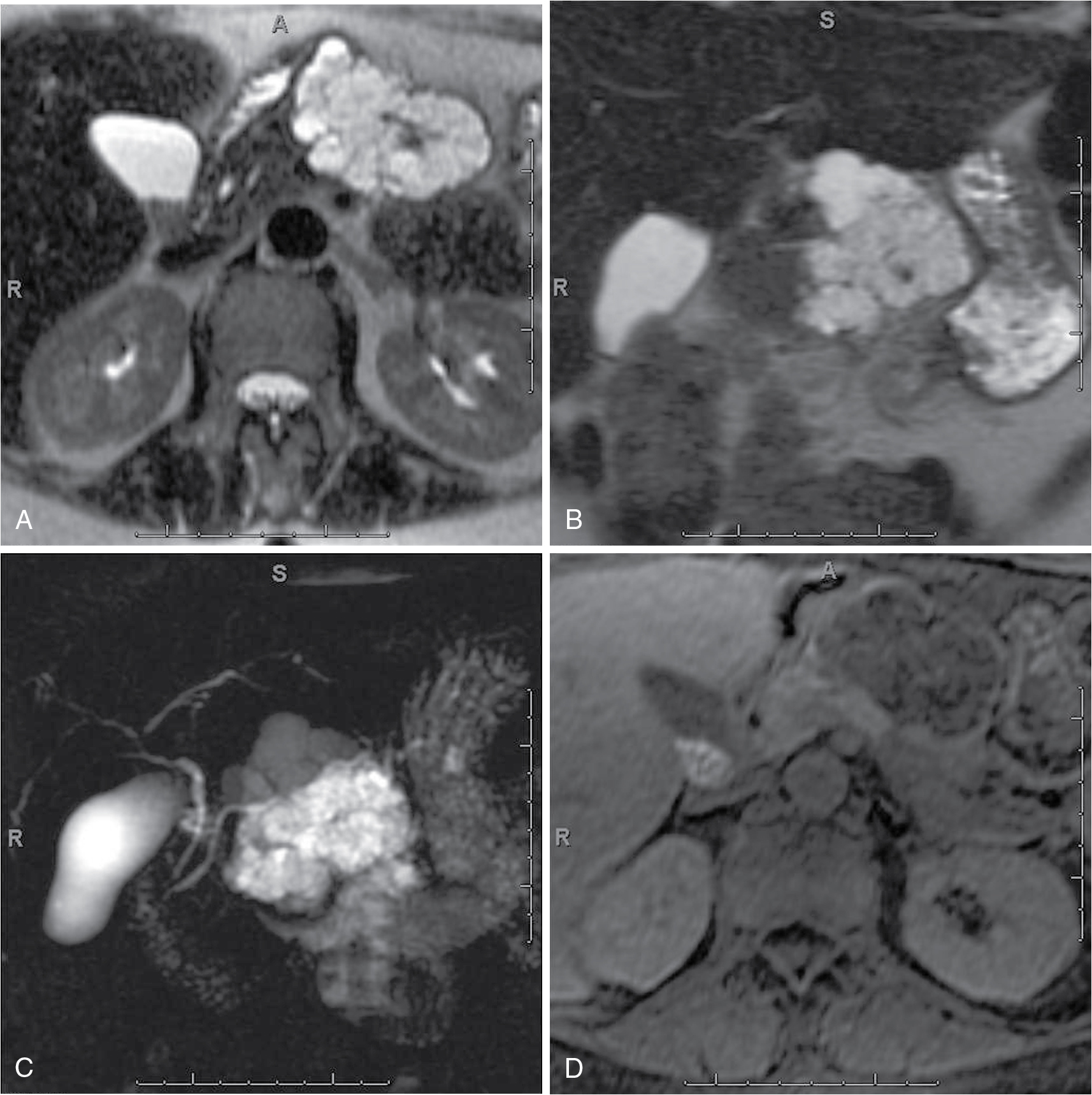
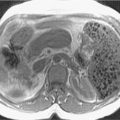
Fig. 14.6
Pancreatic serous cystadenoma. Axial (A), coronal (B), T2-weighted, coronal thick-slab magnetic resonance cholangiopancreatography (C), dynamic precontrast (D), early arterial (E), late arterial (F), and delayed (G) three-dimensional fat-suppressed, T1-weighted, gradient recalled-echo images depict a large multiloculated cystic lesion in the distal body of the pancreas with minimal enhancement of the septations, in keeping with a pancreatic serous cystadenoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree