- •
Delayed-enhancement cardiac magnetic resonance (DE-CMR) can identify reversible myocardial dysfunction before coronary revascularization, predict improvement in contractile function in patients with reperfused acute myocardial infarction, and predict response to ß-blocker therapy in patients with heart failure.
- •
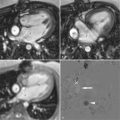
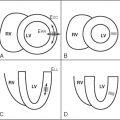
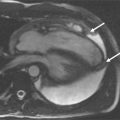
DE-CMR involves T1-weighted imaging of the heart after administration of gadolinium contrast media using a segmented inversion-recovery gradient echo sequence where infarcted or scarred myocardium accumulates gadolinium and appears as “hyperenhanced” or bright.
- •
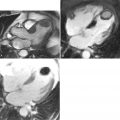
DE-CMR allows the identification of the presence, location, and extent of acute and chronic myocardial infarction with high accuracy relative to histopathology.
- •
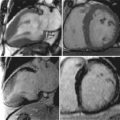
The pattern of hyperenhancement is useful in differentiating ischemic from nonischemic cardiomyopathy. Frequently the specific etiology responsible for nonischemic cardiomyopathy also may be ascertained.
- •
DE-CMR improves the specificity and accuracy of stress perfusion CMR for the detection of coronary artery disease.
- •
Emerging applications for DE-CMR include the detection of intracardiac thrombus and the assessment of patients with intraventricular dyssynchrony for potential cardiac resynchronization therapy.
- •
Myocardial scarring detected by DE-CMR has been associated with adverse prognosis in patients both with ischemic and nonischemic heart disease.
Magnetic resonance imaging of the heart after administration of gadolinium contrast media has been described in the literature for over 20 years. This approach was predicated on the concept that injured tissue accumulates gadolinium and appears as “hyperenhanced” or bright on T1-weighted images acquired at least 10 minutes after injection of gadolinium. A major limitation of the initial approach was poor contrast between normal and injured myocardium. More recently a segmented inversion-recovery gradient echo sequence has been developed that significantly improves in vivo detection of hyperenhanced regions. This technique, known as delayed-enhancement cardiac magnetic resonance (DE-CMR), can identify the presence, location, and extent of both acute and chronic myocardial infarction with high spatial resolution. This technique has been extensively validated with comparisons to histopathology.
Accurate assessment of viable myocardium is important in patients with contractile dysfunction. In these patients if substantial viable myocardium is found, left ventricular function can improve following coronary revascularization and the functional improvement may be accompanied by survival benefits. Identification of nonviable myocardium is also important, because infarcted or scarred tissue may provide a substrate for ventricular tachyarrhythmias and sudden cardiac death. Thus the evaluation of both viable and nonviable myocardium provides important guidance for clinical decision making.
In this chapter we will first illustrate the physiologic basis for DE-CMR, and describe the protocol and typical imaging parameters. Then well-established applications of DE-CMR in patients with ischemic and nonischemic heart disease will be discussed, as well as some newer emerging applications. Finally recent reports demonstrating the prognostic significance of DE-CMR findings will be briefly reviewed.
| PARAMETER | TYPICAL VALUES |
|---|---|
| Gadolinium dose | 0.10-0.20 mmol/kg |
| Field of view | 300-380 mm |
| In-plane voxel size | 1.2-1.8 × 1.2-1.8 mm |
| Slice thickness | 6 mm |
| Flip angle | 20-30 degrees |
| Segments | 13-31 |
| Inversion time (IT) | Variable |
| Bandwidth | 90-250 Hz/pixel |
| Echo time (TE) | 3-4 msec |
| Repetition time (TR) | 8-9 msec |
| Gating factor | 2 |
| K-space ordering | Linear |
| Fat saturation | No |
| Asymmetric echo | Yes |
| Gradient moment refocusing | Yes |