- •
CMR employs multiple techniques in HCM patients to assess LV/RV cardiac function, hypertrophy, morphology, flow, velocities, perfusion, and fibrosis.
- •
Clinical application includes detecting early phenotype expression, characterizing established disease, distinguishing phenocopies, and potentially aiding in risk stratification both for sudden death and heart failure.
- •
In early disease or apical HCM, CMR may detect areas of hypertrophy missed by echo. The presence of LGE may also aid diagnosis.
- •
The extent of LGE is correlated to clinical risk markers for sudden death, progression to heart failure and is predictive of nonsustained VT; thus, LGE may aid in risk stratification.
- •
CMR can be useful in patients with outflow tract obstruction when there are complicating factors such as multilevel obstruction, RV obstruction, aortic membranes, or poor echo windows.
- •
CMR may aid in the diagnosis of phenocopies of HCM.
- •
Patients with progressive disease almost invariably have extensive LGE.
- •
CMR, like all other imaging modalities, has certain pitfalls that are important to be aware of when interpreting scans.
Recent advances in cardiovascular magnetic resonance (CMR) have resulted in its emergence as a useful tool in the evaluation of the patient with hypertrophic cardiomyopathy (HCM) and one that is likely to play an increasing role in the future. The combination of multiple techniques in one scan builds up a sophisticated picture of LV/RV cardiac function, hypertrophy, morphology, flow, velocities, perfusion, and fibrosis.
HCM is a common genetic disease affecting 1 in 500 of the population, manifested by increased myocardial mass, in the absence of loading conditions (hypertension, aortic stenosis) sufficient to cause the abnormality. The most common etiology is an autosomal-dominant disorder caused by mutations in genes that encode cardiac sarcomeric proteins. The pathologic hallmarks of the disease are myocardial hypertrophy, myocyte disarray (usually in association with myocardial fibrosis) and small-vessel disease. Accurate and timely diagnosis relies on several factors including the family history, ECG and echo parameters, and importantly, the screening of families when a proband is identified. Clinical sequelae include systolic and diastolic dysfunction, myocardial ischemia, arrhythmia, abnormal vascular responses, and skeletal muscle dysfunction. Serious complications include thromboembolism, heart failure, infective endocarditis, ventricular arrhythmia, and sudden death. Treatment is designed to reduce symptoms and complications when possible and to prevent disease-related sudden death.
Clinical uses of CMR in HCM include detecting early phenotype expression, characterizing established disease, and distinguishing phenocopies. The potential exists to aid risk stratification both for sudden death and heart failure.
EARLY DISEASE
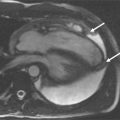
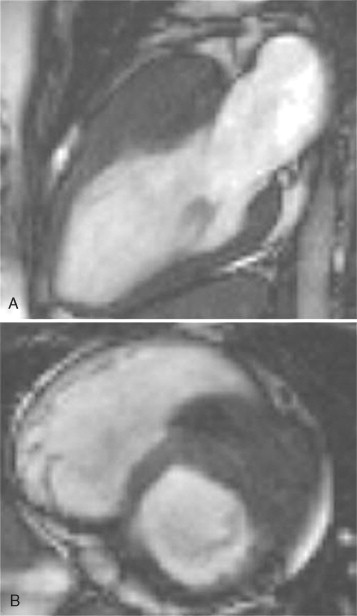
The first signs of phenotypic expression in HCM may be difficult to detect with imaging. In families with HCM, an individual with an affected first degree relative has a 50% pretest probability of gene carriage. The presence/absence of an abnormal ECG is helpful and may strongly suggest disease expression but does not confirm the diagnosis. The traditional gold standard test to assess LV mass—2D echocardiography—has several limitations in this regard. It is reliant on adequate acoustic windows and the fixed probe on the chest wall necessitates oblique short axis cross sectioning of the LV. In addition, although the distribution of hypertrophy in HCM is often diffuse it may frequently be segmental, confined to relatively small regions of the LV chamber that may be obscured or have inadequate border definition on echo. In familial cases, in particular where the ECG is abnormal and the echo normal, cine CMR, which does not have these limitations, may detect missed hypertrophy, occurring in up to 5% of cases in some series ( Figures 11-1 and 11-2 ).
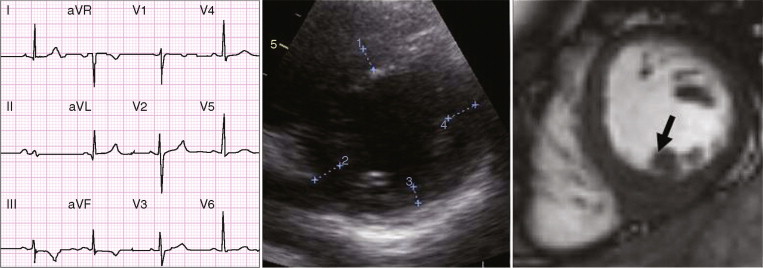
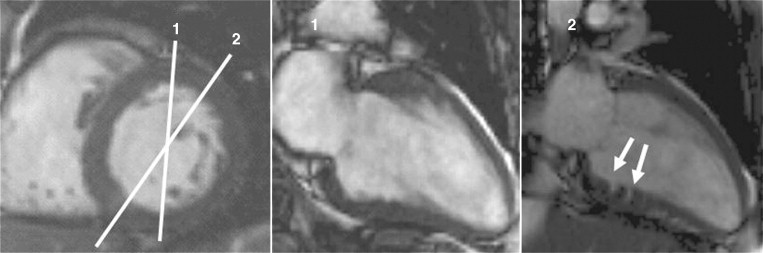
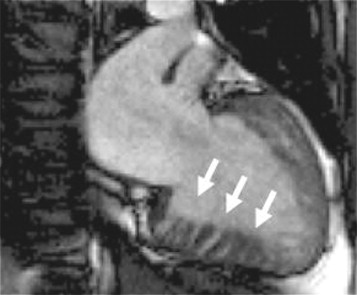
An intriguing suggestion is that there may be pre-phenotypic expressions of sarcomeric protein mutation carriage in minor cardiac morphologic abnormalities—for example crypts (also described as clefts or recesses), particularly at the inferior RV insertion points. Small changes in piloting ( Figure 11-3 ) may reveal these. A few may be part of the normal spectrum, but multiple clefts ( Figure 11-4 ) may be more significant.
APICAL INVOLVEMENT
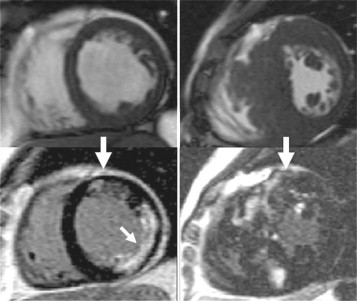
CMR may help the detection of hypertrophy missed by echo. This is particularly true for the apex where echo views are frequently technically challenging because of near field artifact. In patients suspected to have apical hypertrophy (unexplained repolarization abnormalities on ECG, particularly negative T waves anteriorly) CMR may elucidate missed hypertrophy. Additionally, apical cavity obliteration, microaneurysms, thrombus, and LV noncompaction can be identified ( Figure 11-5 ).
PATIENTS AT RISK
Risk stratification in HCM remains challenging. Current risk factors for sudden death include family history of sudden cardiac death (SCD), syncope, nonsustained ventricular tachycardia (NSVT), abnormal blood pressure response to exercise, and severe left ventricular hypertrophy. Applying these risk factors unfortunately does not adequately stratify some patients, who may only have one or no risk factors and still die suddenly. Conversely many patients with defibrillators never require a shock.
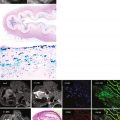
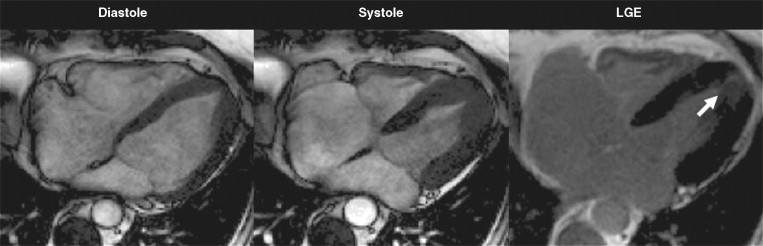
With the use of contrast agents based on gadolinium-DTPA (Gd-DTPA), focal areas of fibrosis can be identified. Gd-DTPA is an extracellular contrast agent and accumulates in areas of scar making the area brighter on CMR—a phenomenon called late gadolinium enhancement (LGE) or delayed enhancement. This is familiar from myocardial infarction where the LGE occurs in coronary artery territories and its distribution in the LV wall reflects the sequence of myocardial ischemia, occurring from the endocardium spreading in a wavefront to the epicardium dependent on the degree of transmurality of the infarct. A similar scarring process occurs in HCM, but the mechanism is less well understood. Correlations have been made between histology and in vivo imaging, showing that LGE, like myocardial infarction, represents increased focal fibrosis rather than disarray ( Figure 11-6 ).
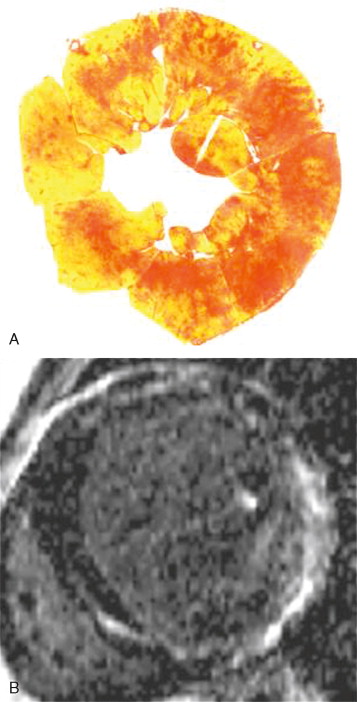
However, in HCM, the extent of LGE and its distribution is different from myocardial infarction and highly variable but does occur in recognizable patterns ( Figures 11-7 and 11-8 ). LGE is common in HCM, occurring in up to 80% of patients, and represents focally increased fibrosis and sometimes complete replacement scarring. Post mortem studies of hearts of patients with HCM have shown greater extent of fibrosis in patients with NSVT. The extent of LGE and hence fibrosis is correlated to clinical risk markers for sudden death, progression to heart failure, and is predictive of NSVT. Therefore, it is plausible that LGE imaging could provide the direct in vivo visualization of the substrate (scar, fibrosis) for sudden death; a highly attractive scenario. The clinical significance of this is not yet fully established and outcome data are needed. Currently extensive LGE in young patients is probably the most concerning finding. It may be most clinically useful in patients possessing a single risk factor for sudden death where clinical management is unclear. The presence of extensive LGE may help those difficult decisions regarding the insertion of a defibrillator.