Diagnostic Techniques
▪ RADIOGRAPHIC TECHNIQUES
Excretory Urography
The importance of excretory urography (intravenous pyelogram [IVP]) as an examination of the urinary tract continues to decline as abdominal computed tomography (CT) and CT urography (CTU) have become the dominant examinations to image urinary tract pathology. Specific indications for excretory urography in adults are few; in most cases, CT or CTU can be used to diagnose any conditions for which IVP was used. IVPs may continue to be performed in situations in which the focus of the study is the renal collecting system or ureters; it should not be done when the suspected pathology is in the renal parenchyma. For this reason, an IVP is not recommended to evaluate patients with hematuria.
Routine Urographic Technique
Bowel preparation is unnecessary if tomography is used or if the clinical situation is urgent. Because intravenous contrast administration may rarely produce vomiting, solid food should be withheld after midnight before the examination. Clear liquids should be encouraged to avoid dehydration, but these should be restricted for an hour or two before the examination to ensure an empty stomach.
There is no universally accepted filming sequence for excretory urography. The best examination is obtained if the study is monitored by the radiologist and modified to answer the clinical question posed. Certain views, however, are essential to every examination. These include a precontrast or “scout” view, a number of delayed excretion views in the anteroposterior (AP) position, and, when possible, thin section tomography to minimize interference from overlying bowel gas.
The Scout Film
The preliminary, or scout, radiograph should extend from the upper renal poles to the symphysis pubis. Calcifications within the urinary tract should be identified before contrast injection because they are often obscured after contrast is excreted. Oblique scout films or tomograms may be obtained to distinguish renal calcifications from extrarenal densities and artifacts.
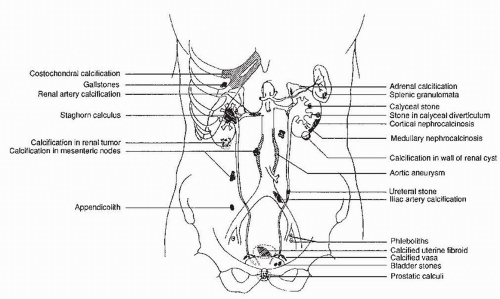
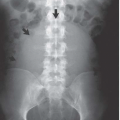
Many common extraurinary calcifications are visible on scout radiographs (Fig. 3.1), including gallstones and pancreatic, nodal, adrenal, costochondral, prostatic, and splenic calcifications. Phleboliths are small, rounded calcifications, often with lucent centers, which are usually located in the pelvis.
Excretion Films
Two or three films of the entire abdomen should be made between 5 and 15 minutes after injection of contrast medium to see the collecting systems, ureters, and bladder. In patients with acute ureteral obstruction, there may be delayed filling of the collecting system and ureter. The amount of delay is highly variable and depends on the acuity and degree of obstruction. The timing of these films is not crucial, but a reasonable schedule would include films at 1, 3, 6, 12, and 24 hours after injection or until the point of obstruction is demonstrated.
Tomography
The use of linear tomography has been considered a standard part of routine urography, as it increases the sensitivity of the urogram for space-occupying lesions and obviates confusing shadows from overlying bowel gas. Tomograms also improve the evaluation of abnormalities of the collecting system, particularly subtle changes in calyceal anatomy and lesions that produce filling defects within the collecting system. However, with the shift to digital imaging techniques, few manufacturers continue to provide linear tomography equipment.
Abdominal Compression
An anterior compression device (often a cloth belt with pneumatic balloons) may be used to compress the ureters against the pelvis so that they are distended and become easily visible proximally (Fig. 3.2). Normal peristaltic activity may otherwise leave portions of the ureters empty of contrast and invisible. However, many authors feel that a nonvisualized segment of a ureter contains noncalcified abnormalities so infrequently that compression is not worthwhile. Contraindications to abdominal compression include an abdominal aortic aneurysm, ureteral obstruction, recent abdominal surgery, and abdominal stomas.
The Normal Excretory Urogram
The findings described next apply not only to conventional excretory urography but also to CT, CTU, or magnetic resonance (MR) urography.
The size of the kidneys varies with the size and sex of the patient; an average renal length equal to three to four vertebral bodies is normal. The average length of the left kidney is approximately 0.5 cm greater than that of the right kidney; size discrepancies are likely to represent abnormalities if the right kidney is more than 1.5 cm longer than the left or if the left kidney is more than 2 cm longer than the right.
The kidneys lie in the retroperitoneum with their long axes parallel to the outer border of the psoas muscles. The renal hilus is usually at the same level of the L2 or L3 vertebral body. The normal kidney should be sharply marginated and smooth in outline. Wellperformed urography should permit visualization of the entire outline of each kidney; failure to visualize a portion of the outline may be the only manifestation of a focal abnormality.
Fetal lobation is a normal variant in which the renal margin is lobulated. The indentations between the lobes represent incomplete fusion of the embryologic renal lobules. These indentations can be differentiated from pathologic scarring by their smooth contour and regular spacing. They can be distinguished from pyelonephritic scars, which are usually deeper and are always adjacent to an underlying abnormal calyx. Scars from renal infarcts occur in random locations. A prominent bulge on the lateral border of the left kidney represents a normal variation because of molding by the adjacent spleen and has been called a dromedary hump. The average human kidney contains 10 to 15 calyces, but this number may vary considerably. Calyces in the polar regions, particularly in the right upper pole, may be compound; that is, two or more papillae may share a common infundibulum. Normal calyces are delicately cupped without blunting or distortion. Normal infundibuli are straight and demonstrate neither bowing nor displacement. Occasionally, a small blush of contrast may be seen in the renal papilla immediately adjacent to the calyx, which is related to the relatively high concentration of contrast material in the distal collecting ducts. This finding is especially frequent in thin patients and should not be confused with the dilation of the distal collecting ducts seen in medullary sponge kidney.
In older patients, the calyces and renal pelvis may be compressed by the accumulation of fat in the renal sinus. This condition, termed renal sinus lipomatosis, may produce an attenuated appearance of the collecting system and may prevent adequate calyceal distention, but it carries no clinical significance. Frequently, segmental arteries or veins produce impressions on the infundibuli of the major or minor calyces; these defects are sharply marginated, and when produced by crossing veins, may be eliminated with tight abdominal compression. An extrinsic impression on the infundibuli or renal pelvis may be produced by a large column of Bertin,
which is a region of cortical tissue between two medullary pyramids; this finding is most common at the junction of the superior and middle thirds of the kidney.
which is a region of cortical tissue between two medullary pyramids; this finding is most common at the junction of the superior and middle thirds of the kidney.
The major calyces join to form the renal pelvis, whose appearance varies considerably from patient to patient. Often the pelvis is entirely intrarenal, but sometimes it appears to be outside the confines of the kidney, where it often has a distended appearance (the extrarenal pelvis).
The ureters are continuous with the renal pelvis approximately at the level of the second lumbar vertebra; they descend through the cone of renal fascia just lateral to the transverse processes of the upper lumbar vertebrae. The middle third of the ureter is usually superimposed on the transverse processes of the lower lumbar vertebra; considerable normal variation in the ureteral course may occur. The ureter is most anterior where it passes anterior to the iliac vessels; it then descends into the pelvis, coursing first posterolaterally and then anteromedially to enter the bladder at the trigone. Regular waves of peristalsis course down the ureter; they begin in the renal pelvis and, if normal, completely coapt the walls of the ureter so that lengthy segments of the ureteral lumen may be invisible on particular radiographs.
One or more slight constrictions of the ureter may sometimes be visualized at the level of the ureteropelvic junction. These are transverse folds and are normal (Fig. 3.3). Other normal urographic features may include indentations as the ureters are crossed by the ovarian vessels, and as they cross the iliac vessels, particularly in older patients. In younger male patients, ureters may appear to be displaced as they course anterior and medial to large psoas muscles (Fig. 3.4).
The size and contour of the bladder vary with the degree of filling. When distended, the normal bladder is roughly spherical. The superior portion may be indented by overlying viscera; in particular, the uterus frequently indents the bladder dome. Any straightening or deformity of one of the contours of the bladder should be considered a sign of mural abnormality unless an extravesical structure can be identified as causing the indentation. On a postvoid radiograph, the mucosal pattern of the bladder should be identified.
Retrograde Pyelography
Retrograde injection of contrast material directly into the ureter allows visualization of the collecting system and ureter without relying on the kidney to excrete contrast medium. The degree of opacification and distention of the collecting system can be controlled by varying the volume and concentration of the injected contrast medium. It should only be performed if satisfactory
evaluation of the collecting system cannot be achieved by a less invasive method. To perform the examination, the ureteral orifice is cannulated cystoscopically. The catheter may be advanced to the renal pelvis, or the contrast material may be injected into the distal ureter. The examination is best performed with fluoroscopic monitoring and appropriate spot films, augmented by overhead views when necessary. When a urothelial lesion is suspected, brushing or biopsy of the lesion for a pathologic specimen may be performed.
evaluation of the collecting system cannot be achieved by a less invasive method. To perform the examination, the ureteral orifice is cannulated cystoscopically. The catheter may be advanced to the renal pelvis, or the contrast material may be injected into the distal ureter. The examination is best performed with fluoroscopic monitoring and appropriate spot films, augmented by overhead views when necessary. When a urothelial lesion is suspected, brushing or biopsy of the lesion for a pathologic specimen may be performed.
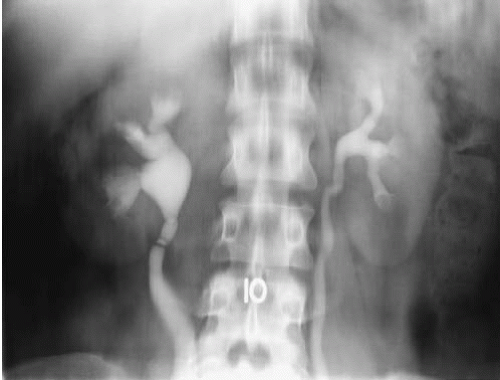 FIGURE 3.3. Transverse ureteral folds. Several band-like constrictions that have no physiologic or pathologic consequence are present in the right proximal ureter. |
 FIGURE 3.4. The ureters have a more medial course secondary to large psoas muscles in this young male patient. This condition is sometimes referred to as psoas hypertrophy. |
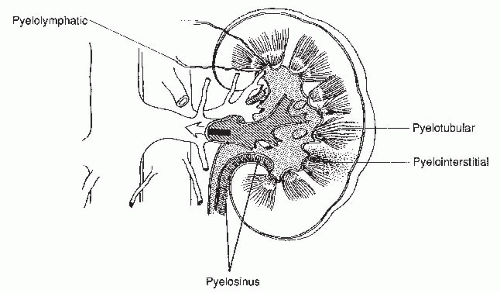
There are several potential complications of retrograde pyelography. The cannulated ureteral orifice may become edematous and transiently obstructed. The pyelocalyceal system may be overdistended, so that contrast escapes from small ruptures in the fornices into the renal sinus; this is known as pyelosinus backflow. If the contrast then opacifies the renal vein or lymphatics, it is known as pyelovenous or pyelolymphatic backflow. Contrast injected under high pressure into the collecting system may also flow retrograde into the renal tubular lumina, and this is known as pyelotubular backflow (Fig. 3.5). These occurrences are usually insignificant, but if the patient has a urinary infection, absorption of infected urine may produce systemic sepsis.
Cystography
Static cystography is performed to assess suspected bladder rupture, vesical fistula, and low-pressure vesicoureteral reflux. Using a Foley catheter, 300 to 400 mL of contrast material (20% to 30% weight per volume) is instilled into the bladder, and radiographs in the AP, oblique, and lateral positions are obtained. A postdrainage view is also obtained and is considered a mandatory part of a trauma cystogram, as small amounts of contrast material extravasation may be hidden behind a distended bladder. When assessing possible vesicovaginal fistulae, imaging the lateral projection should be obtained.
Voiding cystourethrography is used to diagnose high-pressure vesicoureteral reflux and to evaluate the urethra. The bladder is filled with contrast material using a Foley catheter as for a static cystogram. The bladder should be filled until the patient is certain that he or she can void when the catheter is removed, and voiding cystography is performed with fluoroscopy. High-pressure reflux, bladder extravasation, or a small-necked diverticulum may not be apparent until the voiding study is obtained. In female patients, AP radiographs of the urethra are adequate. In male patients, however, the voiding films should be obtained in a 45° oblique position, so that the entire length of the urethra is demonstrated. Some films should be centered on the bladder, particularly when vesicoureteral reflux is suspected. If reflux is present, the highest level of that reflux should be documented on spot radiographs.
Ileal Conduit Studies
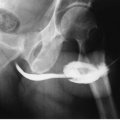
After cystectomy, an ileal conduit is commonly constructed for supravesical urinary diversion. One end of an isolated segment of ileum is brought to the skin as an ileocutaneous stoma. The ureters are implanted in the opposite end of the loop; there is generally ready reflux from the conduit into the ureters. Contrast material instilled into the ileal loop may demonstrate the ureters and the renal collecting systems (Fig. 3.6). Such a study is called an ileal conduit study or a “loopogram.”
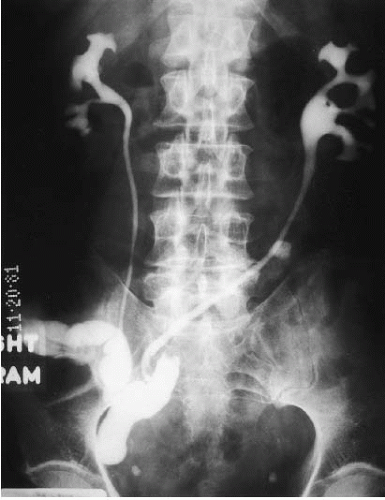 FIGURE 3.6. Loopogram. Contrast material is instilled through a Foley catheter into the ileal conduit and refluxes up both ureters, thereby demonstrating the renal collecting system. |
To perform a loopogram, an appropriate Foley catheter (generally between a 20- and 26-French) is inserted into the stoma and the balloon is inflated to a volume of 5 to 8 mL within the stoma. Contrast material is then instilled through the Foley catheter by gravity infusion or hand injection. Gravity infusion is generally preferred, so that the pressure will be limited to the height of the bottle of contrast material. Under fluoroscopic observation, contrast material fills the loop and will usually reflux into the ureters. The study is especially useful in patients with impaired renal function and serves to exclude ureteral obstruction.
Modern urinary diversion is frequently performed by fashioning an internal reservoir that stores urine and can be emptied periodically by catheterizing a continent stoma, or in selected patients attached to the urethra allowing the patient to void normally. These reservoirs, which are also known as neobladders, continent diversions, or pouches, may be orthotopic (i.e., located at the site of a resected bladder) or may be located near the anterior abdominal wall. They are usually constructed so that reflux from the reservoir into the ureters is prevented.
To examine the neobladder, the stoma should be carefully catheterized with a small Foley catheter and the balloon slowly inflated; inflation should cease as soon as gentle traction on the catheter can be performed without removing it. Rupture of any portion of the neobladder by distending the balloon in a narrow channel leading to the stoma is a complication to be avoided. The reservoir should be emptied as much as possible through the catheter, after which contrast should be injected under low pressure and using fluoroscopic control. Spot films in posteroanterior, lateral, and oblique projections should be obtained, and the presence or absence of anastomotic leaks or reflux should be documented. The catheter should be removed without emptying the reservoir; the patient should then perform self-catheterization before a final film is obtained to see how well the reservoir is emptied. Scout films should precede these examinations so that faintly calcified stones will not be overlooked.
Urethrography
The male urethra may be visualized by filling it with contrast in a retrograde or antegrade fashion. Retrograde urethrography and voiding cystourethrography may be performed separately, but to evaluate the entire male urethra, both should be performed.
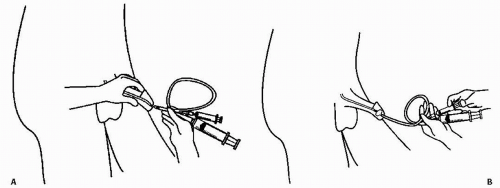
To perform retrograde urethrography, the distal urethra should be occluded by a small Foley catheter. A 12- or 14-French Foley is inserted into the urethra until the deflated balloon just disappears inside the meatus; the balloon is inflated with 1 to 2 mL of saline so that it distends in the fossa navicularis (Fig. 3.7). Inflation should be performed slowly and carefully and terminated as soon as mild traction on the catheter does not remove it from the urethra. The catheter should be flushed to remove air bubbles before insertion into the meatus.
Full- or half-strength contrast should be injected using a 50-mL syringe under fluoroscopic control. The patient lies in a supine oblique position with the dependent hip flexed; the penis is positioned pointing anteriorly along the flexed leg. Simultaneous
fluoroscopy and injection are performed until the entire anterior urethra is filled and radiographed. Ideally, the study should be dynamic; that is, filming should be performed as contrast is injected to distend the anterior urethra. Injection should continue until a small amount of contrast traverses the posterior urethra into the bladder.
fluoroscopy and injection are performed until the entire anterior urethra is filled and radiographed. Ideally, the study should be dynamic; that is, filming should be performed as contrast is injected to distend the anterior urethra. Injection should continue until a small amount of contrast traverses the posterior urethra into the bladder.
Retrograde urethrography demonstrates the anterior urethra. Even if contrast is injected under sufficient pressure to traverse the posterior urethra, the posterior portion is not distended because the sphincters are contracted. To demonstrate the posterior urethra, voiding urethrography should be performed.
Voiding urethrography is usually performed after the bladder has been filled with contrast instilled through a urethral catheter, although it may also be performed by voiding opacified urine produced during an excretory urogram. The former method is preferable because the contrast is much more opaque. If the patient voids around a small-caliber catheter (which is usually performed without difficulty, provided there is no inflated Foley balloon to occlude the internal meatus), the bladder can be refilled and the patient may void several times, if necessary.
For voiding urethrography, the bladder is filled with several hundred milliliters of contrast, or is filled until the patient feels bladder distention and an urge to void. The patient ideally voids in an upright position, although the study may be done with the patient on a horizontal table. In any case, male patients should be imaged in a 45° oblique position; females should be examined in a posteroanterior position. Fluoroscopic control should be performed, and several views centered on the posterior urethra should be obtained during maximal voiding.
It may occasionally be necessary to perform urethrography in women by injecting contrast directly into the urethra. This procedure may be useful to diagnose urethral diverticula, which may not opacify during voiding, or to demonstrate fistulae from the urethra. A special catheter, sometimes known as a double-bubble catheter, is necessary for this procedure. The catheter has a distal balloon, much like that on a Foley catheter, and an external balloon, which may either be fixed or slide along the catheter and which is used to seal the external meatus. There is an opening between the two balloons to inject contrast into the urethra. The catheter is inserted, the internal balloon inflated, and the catheter is then withdrawn so that the internal balloon is firmly seated at the internal meatus and the external balloon is inflated and pressed firmly against the external meatus. Several milliliters of contrast are then injected under fluoroscopic control; contrast distends the urethra under pressure and will fill any diverticulum or fistula that extends from it.
▪ ULTRASONOGRAPHY
Renal Sonography
There are a variety of uses for renal ultrasound. It is especially valuable in children and pregnant women where exposure to ionizing radiation should be minimized. Because image display is immediate, it is often used as a guidance technique for a variety of invasive procedures, including antegrade pyelography, percutaneous nephrostomy, percutaneous abscess drainage, percutaneous renal biopsy, and tumor ablation.
Limitations to renal sonography include obese patients, those with abdominal dressings, and those with a large amount of intraabdominal air, who may be difficult to examine. The quality of the examination varies tremendously with the experience of the sonographer. Finally, with the exception of assessment of flow velocity by Doppler examination, renal ultrasound remains a morphologic, rather than a functional, technique.
The kidneys are usually examined with the patient in the supine or supine oblique position. Anterior or anterolateral transducer positions enable the right kidney to be well visualized through the liver and the left kidney through the spleen. Deep inspiration may move the kidneys inferiorly for improved visualization; occasionally, an intercostal transducer position may be useful. The prone position is rarely used, but may occasionally provide reasonable visualization of the kidneys.
Transducer frequency should be chosen to match the patient’s habitus: the frequency should be as high as possible, but still permit detailed visualization of renal structures. Static images should document all aspects of both kidneys in the axial and transverse planes. If the reason for examination requires visualization of particular features, these should be documented, even if normal.
Ultrasound contrast agents may help overcome some of the inherent limitations of sonography by increasing the sensitivity of the technique to changes in blood flow and by increasing the inherent contrast of small renal masses. Most agents are based on microbubble technology and are thus highly echogenic. Although these agents have been approved in Europe, none is currently approved in the United States for routine use.
Intraoperative ultrasound may be used to localize nonpalpable intrarenal lesions in the operating room. This is a particularly useful technique with today’s nephron-sparing surgical procedures.
Doppler Sonography
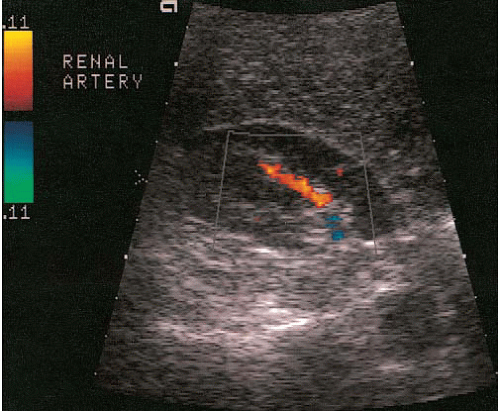
Three types of Doppler ultrasound instruments are available: continuous wave devices, which are seldom used in radiologic practices; pulsed duplex; and color flow instruments. Pulsed-duplex devices allow the display of flow in a small area on a corresponding gray-scale image as a continuous time-velocity wave-form (Fig. 3.8). With a color flow device, a color-encoded Doppler signal is superimposed on a gray-scale image, in which red is used to indicate flow toward the transducer (Fig. 3.9) and blue to indicate flow away from the transducer; paler and deeper hues represent higher and lower velocities, respectively. A color flow device can display the flow over a larger area of the image, unlike the pulsed-duplex device. A modification of standard color Doppler ultrasound, called “power Doppler,” offers extended dynamic range (albeit at the loss of directional information) and can demonstrate tissue perfusion that a standard color Doppler image might not visualize.
Applications of Doppler imaging in uroradiology include the evaluation of renal transplants; the search for perfusing vessels in space-occupying renal lesions; the detection of arteriovenous fistulae; the assessment of renal artery stenosis and occlusion; the detection of renal vein thrombosis; the evaluation of suspected scrotal lesions such as torsion, tumors, inflammation, and varicoceles; and the evaluation of ureteral obstruction by assessing intravesical ureteral jets (Fig. 3.10).
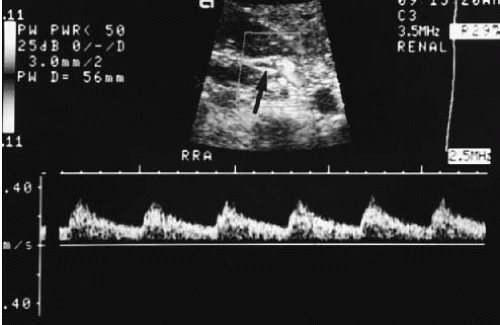 FIGURE 3.8. Pulsed duplex ultrasound. The Doppler gate is positioned over the renal artery (arrow). The resulting waveform is displayed below the gray-scale image. |
Bladder Sonography
The bladder should be filled for optimal examination; this can usually be accomplished with oral fluids and voiding restriction, but may require bladder filling through a catheter in anuric patients. The transducer is placed in the suprapubic region and angled appropriately for representative axial and transverse views of the bladder. The gain control should be adjusted to make urine anechoic.
Bladder ultrasound may detect bladder stones, foreign bodies, and diverticula, and may be used to assess prostate size. Bladder tumors may also be identified, but ultrasound is not sufficiently sensitive in detecting small flat tumors to exclude a bladder neoplasm. Extravesical fluid collections and masses may also be demonstrated. The bladder volume may be reasonably well approximated from width, length, and depth measurements using the following formula:
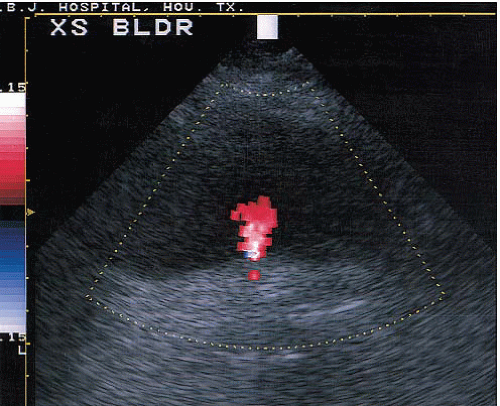 FIGURE 3.10. Ureteral jet. A transverse image of the bladder showing a jet of urine (red) from the ureteral orifice. |
Bladder volume = 0.7 (W × H × D)
Such estimation of bladder volume is accurate within approximately 20%, which is considered acceptable; it is most often used to estimate postvoid residual volumes.
▪ PROSTATE SONOGRAPHY
Ultrasound of the prostate is most frequently used as a biopsy guide for patients in whom digital rectal examination or prostatespecific antigen (PSA) levels suggest prostate carcinoma. It is no longer used as a purely diagnostic modality and has not been found to be sufficiently sensitive or specific to screen for prostate cancer. As a biopsy guide, it is used both to guide the biopsy device into specific sections of the prostate whose ultrasound appearance suggests tumor and to assure that random biopsies sample tissue from multiple separate areas of the gland. It is useful to detect seminal vesicle masses and cysts. It can also be used to diagnose less common prostate conditions, such as abscesses, cysts, and utricular abnormalities.
Prostate ultrasound is performed with an intrarectal probe. Transducers are high frequency (often 7 MHz) and should be able to provide both transverse and longitudinal images from the same probe. A series of transverse images that extend from the base of the prostate and seminal vesicles inferiorly to the apex are obtained. Sagittal and angled sagittal images should also be acquired to display the gland in longitudinal views.
The focal abnormalities, which may represent prostate carcinoma and which are biopsied using transrectal ultrasound guidance, are usually in the peripheral zone of the prostate, where most prostate cancers arise, and are usually relatively hypoechoic with respect to surrounding tissue.
Gynecologic Sonography
Gynecologic ultrasound may be performed either transabdominally or transvaginally. Transperineal and rectal probes have been used for special circumstances, but are not routine procedures.
In each case, the patient is examined supine. Occasionally, slight elevation of the upper body improves pelvic visualization if there is ascitic fluid that may pool in the pelvis. The legs are comfortably straight for transabdominal examinations and in a lithotomytype position for transvaginal scans. The bladder should be filled to act as an acoustic window for transabdominal examinations, and empty to reduce discomfort for transvaginal sonography.
For most adults, transabdominal examinations are best begun with a 3.5-MHz probe; for relatively small patients and superficial structures, higher frequencies may be used. Transvaginal probes usually operate within the 5- to 7.5-MHz range.
Multiple views are required for complete gynecologic examination. Variations in body habitus, uterine orientation, and ovarian location and mass size determine the best combination of transducer locations, imaging plane directions, and orientations. For transabdominal ultrasound, sagittal, parasagittal, and angled sagittal views are often useful to demonstrate the uterus along its main axis. Transverse and angled transverse views depict the uterus in various sections across its main axis; clearly, transducer location and direction and uterine position and flexion offer a large variety of combinations for longitudinal and axial views of the uterus. The adnexal regions are often seen best in transverse views; the transducer may be rotated for angled or sagittal views that include adnexal structures once they have been identified. If masses originating in the pelvis extend cephalad to any degree, the transducer must be moved along the abdominal surface to construct views of the entire structure.
During transvaginal ultrasonography, sagittal views are often useful to demonstrate the uterus along its longitudinal axis. Positioning the transducer head in the vaginal fornix often permits visualization of the uterine fundus and body; withdrawing the transducer head slightly may permit better visualization of the lower uterine segment and cervix. The position of the uterus and adjacent structures may be changed by abdominal palpation to move them into a better position for transvaginal depiction. As the transducer is rotated 90° to produce a coronal or angled coronal view, the adnexal regions may be scanned more easily; the ovaries can often be detected by angling the stem of the transducer to each side and by angling the coronal imaging plane so that it sweeps anteriorly to posteriorly and scans through the entire adnexal region.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree