Diaphragm
Mark C. Liszewski
Pedro Daltro
Celia Ferrari
Gloria Soto Giordani
Fred E. Avni
Edward Y. Lee
INTRODUCTION
The diaphragm is a musculofibrous structure that separates the thoracic and abdominal cavities and is integral to respiratory function. In the pediatric population, several important pathologic conditions can affect the diaphragm, which include congenital and developmental anomalies, neoplasms, and trauma. In this chapter, imaging techniques to evaluate the diaphragm are discussed, the normal anatomy of the pediatric diaphragm is reviewed, and the spectrum of diaphragmatic disorders is presented.
IMAGING TECHNIQUES
Several currently available imaging modalities, alone or in combination, can be used to effectively evaluate the diaphragm in infants and children. Each modality’s utility depends largely on the pathologic condition in question. The current imaging techniques of various imaging modalities used for evaluating the diaphragm are discussed in the following section.
Radiography
Chest radiography often serves as the first-line imaging modality to evaluate the diaphragm. Whenever possible, both frontal and lateral chest radiographs are recommended for a complete assessment of the diaphragm in pediatric patients. Normally, the upper aspect of the diaphragm is seen adjacent to the aerated lung, and the lower aspect is silhouetted by the abdominal viscera on frontal chest radiograph (Fig. 13.1).1 The position of the diaphragm is determined by the phase of respiration at the time of imaging. When imaging at end inspiration, the superior aspect of the right hemidiaphragm normally projects at approximately the level of the anterior sixth rib,2 and the dome of the left hemidiaphragm projects approximately one intercostal space lower.1 However, there is wide variability in the position of the normal diaphragm.3 On the lateral radiograph, the domed right hemidiaphragm is seen in its entirety, whereas the anterior left hemidiaphragm is silhouetted by the mediastinum superiorly (Fig. 13.2).1
Although radiographs do not provide detailed information about the diaphragm, secondary radiographic signs may suggest a pathologic condition of the diaphragm and can guide the appropriate next imaging studies for further evaluation. For example, herniated abdominal viscera may be seen within the thorax in cases of congenital diaphragmatic hernia (CDH), though the actual diaphragmatic defect may be poorly visualized radiographically. Similarly, persistent elevation of the diaphragm may suggest a diagnosis of diaphragmatic paralysis, though other modalities, such as ultrasound (US) and fluoroscopy that can provide real-time assessment, are often needed for definitive diagnosis.
Ultrasound
Because of its ability to visualize structures in real time without the use of ionizing radiation, US is an attractive and useful noninvasive imaging modality in the evaluation of the diaphragm. Curved or linear array US transducers are typically used for evaluation of the diaphragm. Higher-frequency US transducers (7.5 to 15.0 MHz) provide higher-resolution images but have less soft tissue penetration. Therefore, high-frequency transducers are often the best tool for evaluating the diaphragm in infants and young children with small amounts
of subcutaneous fat but can be less helpful in larger children with less optimal acoustic windows. Lower-frequency US transducers (<5 MHz) produce lower-resolution images but provide better soft tissue penetration and are more suitable for larger children with greater amounts of subcutaneous fat.4
of subcutaneous fat but can be less helpful in larger children with less optimal acoustic windows. Lower-frequency US transducers (<5 MHz) produce lower-resolution images but provide better soft tissue penetration and are more suitable for larger children with greater amounts of subcutaneous fat.4
The diaphragm is a specular reflector and is seen as an echogenic linear structure on US (Fig. 13.3). Each hemidiaphragm can be imaged individually in axial, sagittal, and/or oblique planes, or both hemidiaphragms may be visualized simultaneously utilizing a transverse subxiphoid view in infants and young children with an available acoustic window.1 US is particularly useful in the evaluation of diaphragmatic paralysis, as it can visualize movement in real time. The use of M-mode imaging allows for accurate quantification of this movement.5,6 High spatial resolution and portability make US particularly useful in the evaluation of diaphragmatic hernias7 and eventration.5,8 US may also be helpful in selected cases of late-presenting CDH9 and traumatic diaphragmatic rupture,10,11 though a multimodality approach is often employed in the diagnosis of these conditions. Additionally, US may be useful in the evaluation of diaphragmatic tumors, though localization of the mass to the diaphragm as opposed to an adjacent abdominal organ is often challenging without additional cross-sectional imaging assessment.12,13
Fluoroscopy
Before the increased use of US, fluoroscopy was the primary imaging tool used to evaluate diaphragmatic movement, particularly in the pediatric population. In selected cases, fluoroscopy can be a useful tool to evaluate diaphragmatic motion when US may be unavailable or suboptimal. Imaging can be performed during quiet breathing, deep breathing, coughing, and/or sniffing in infants and children in frontal and lateral projections. As with all studies
that utilize ionizing radiation, the ALARA (As Low As Reasonably Achievable) principle of radiation dose management should be closely followed.14 Helpful methods that can be employed to reduce overall radiation dose during fluoroscopy include (1) removing the antiscatter grid when imaging small patients (<18 kg and <4 years old), (2) using the largest fields of view (i.e., smallest electronic magnification) possible, and (3) collimating to the area of interest.14 Diaphragmatic paralysis is suggested when there is absent or paradoxical movement of a hemidiaphragm. Diaphragmatic eventration may be suggested when there is a lag at inspiration, followed by delayed inferior movement, though differentiation from diaphragmatic paralysis is often challenging.
that utilize ionizing radiation, the ALARA (As Low As Reasonably Achievable) principle of radiation dose management should be closely followed.14 Helpful methods that can be employed to reduce overall radiation dose during fluoroscopy include (1) removing the antiscatter grid when imaging small patients (<18 kg and <4 years old), (2) using the largest fields of view (i.e., smallest electronic magnification) possible, and (3) collimating to the area of interest.14 Diaphragmatic paralysis is suggested when there is absent or paradoxical movement of a hemidiaphragm. Diaphragmatic eventration may be suggested when there is a lag at inspiration, followed by delayed inferior movement, though differentiation from diaphragmatic paralysis is often challenging.
Computed Tomography and Magnetic Resonance Imaging
Because of its excellent contrast resolution and multiplanar imaging capability, computed tomography (CT), particularly multidetector CT (MDCT), and magnetic resonance imaging (MRI) are both excellent tools for evaluating the diaphragm.1,8,15 Additionally, specialized MR imaging can be performed to evaluate diaphragmatic motion by employing fast gradient-recalled-echo (GRE) pulse sequences16 or utilizing steady-state acquisition sequences (FIESTA; GE Medical Systems).17 The excellent soft tissue resolution of MRI allows the diaphragm to be visualized as a thin muscular sheet that is lower in signal intensity than other skeletal muscles on all sequences (Fig. 13.4).1
CT and MR can be useful in evaluating pathologic conditions of the diaphragm. For example, CT and MRI are helpful in the diagnosis and management of diaphragmatic tumors, though attributing an origin from the diaphragm as opposed to an adjacent abdominal organ can be challenging.12,13 CT is useful in the diagnosis of traumatic diaphragmatic injury.10 The large majority of CDHs are diagnosed and managed based on antenatal US and MRI findings as well as postnatal radiographs, but in selected cases, CT or MRI can provide important information about hernia contents, particularly in cases of late presentation or when findings are unclear.1,8,9
NORMAL ANATOMY AND DEVELOPMENT
The diaphragm begins development in the 4th week of gestation.7 Bilateral pleuroperitoneal membranes fuse with the horizontal septum transversum, the mesentery of the esophagus, and the musculature of the lateral body wall to form the diaphragm.18 The septum transversum forms the central tendon, which is the central fibrous portion of the diaphragm (Fig. 13.5). The majority of the muscular diaphragm is derived from the pleuroperitoneal membranes, with the peripheral portions derived from the lateral body wall and midline portions, including the crura, formed by the dorsal
mesentery of the esophagus.1 A fibrous lumbocostal trigone remains at the posterior lateral junction of each hemidiaphragm, where the pleuroperitoneal membranes fuse with the lateral body wall muscle fibers.7
mesentery of the esophagus.1 A fibrous lumbocostal trigone remains at the posterior lateral junction of each hemidiaphragm, where the pleuroperitoneal membranes fuse with the lateral body wall muscle fibers.7
The mature diaphragm is characterized by anterolateral muscle fibers that originate from the sternum and ribs and posteromedial fibers that originate from the right aspect of the upper three lumbar vertebrae and the left aspect of the upper two lumbar vertebrae as the crura.1,18 The three major openings in the normal diaphragm are the aortic hiatus, esophageal hiatus, and inferior vena caval hiatus. The aortic hiatus is at the level of T12 and contains the aorta, thoracic duct, azygos vein, and hemiazygos vein.18 The esophageal hiatus is at the level of T10 and contains the esophagus, vagus nerve, sympathetic nerves, and esophageal branches of the left gastric vessels.18 The inferior vena caval hiatus is located at the level of T8-9 and contains the inferior vena cava and branches of the right phrenic nerve.18
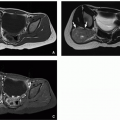
The anterior diaphragm can have a variable appearance on cross-sectional imaging depending on the position of the middle leaflet of the central tendon relative to the xiphoid.19 Familiarity with the normal variations in diaphragmatic morphology is essential, particularly when evaluating for Morgagni hernias.19 Three normal configurations of the anterior diaphragm have been described (Fig. 13.6).1,18,19 In type I, the middle leaflet of the central tendon is located superior to the xiphoid process with posterior concavity of the anterior portion and continuity between the anterior and anterolateral fibers (Fig. 13.6A, B). In type II, the middle leaflet of the central tendon is located inferior to the xiphoid process, and the anterior diaphragm is discontinuous in the midline, with the anterior fibers oriented at an angle to the lateral fibers (Fig. 13.6C, D). In type III, the middle leaflet of the central tendon and the xiphoid process are at the same level causing the anterior diaphragm to appear broad and angular on axial images with poorly defined margins because it runs in the plane of the axial image (Fig. 13.6E, F). M. Elon Gale described the incidence of each configuration, finding type I in 48%, type II in 28%, and type III in 11%.19
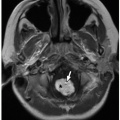
 FIGURE 13.7 A 28-month-old girl with a normal diaphragm. Axial enhanced CT image shows a normal nodular appearance of bilateral diaphragmatic crura (arrows). |
Familiarity with the normal appearance of the diaphragmatic crura is also essential to avoid potential delayed diagnosis or misdiagnosis. Because crural width does not change substantially with age, the crura appear relatively larger in children.20 This may lead the normal crura to have a more nodular appearance in the pediatric patient population, which should not be mistaken for pathology (Fig. 13.7).
SPECTRUM OF DIAPHRAGMATIC DISORDERS
Congenital and Developmental Anomalies
Three main congenital anomalies of the diaphragm include diaphragmatic hernias, diaphragmatic eventration, and duplication of the diaphragm (Table 13.1). These conditions are encountered with varying frequency, and familiarity with these anomalies is essential to avoid confusion and misdiagnosis.
Diaphragmatic Hernia
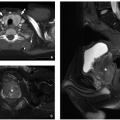
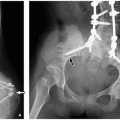
Diaphragmatic hernias can occur as a result of a defect in the diaphragmatic musculature, as in CDH, or because of protrusion of the stomach through the esophageal hiatus, as in hiatal hernia. CDHs are potentially serious congenital anomalies with a high morbidity and mortality and frequent associations with additional malformations, syndromes, and chromosomal anomalies.7 CDH occurs in ˜1 in 4,000 births21 and is traditionally classified based on location. Posterolateral hernias that pass through the foramen of Bochdalek (i.e., Bochdalek hernias [BHs]) are the most common type, accounting for ˜90% of all CDHs (Fig. 13.8).22 Parasternal hernias that pass through the foramen of Morgagni (i.e., Morgagni hernias) are the next most common type, accounting for 9% to 12% of CDHs (Figs. 13.9 and 13.10).7
TABLE 13.1 Congenital Anomalies of the Diaphragm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
A recent classification scheme proposes new categories and definitions for diaphragmatic hernia and eventration and provides opportunities for more detailed subtyping based on such parameters as size, location, presence, or absence of a “sac” or dividing membrane and other anatomic factors.23 Improving the precision of phenotypic diagnosis may pave the way for better genotype-phenotype correlation as more genetic causes of CDH are understood. However, many of the detailed parameters involved in the new classification scheme are better addressed by the type of gross inspection available at surgery and autopsy than by radiographic study, and this classification scheme has not been widely adopted by the radiology community to date. Nevertheless, the limitations of categorizing the traditional “Bochdalek” and “Morgagni” hernias are recognized, and therefore, the following classifications should be utilized only as a guideline for interpretation.
Bochdalek Hernia
The pathogenesis of Bochdalek hernias (BHs) is poorly understood. It has been suggested that the primary inciting event in this condition is failure of the septum transversum and pleuroperitoneal fold to fuse with the musculature of the lateral body wall at ˜8 weeks of gestation, though more recent studies have shown that the defect may occur earlier, during the development of the pleuroperitoneal folds.24 Because Bochdalek hernia (BH) is associated with additional malformations of the heart, lungs, and other organ systems, it has been postulated that the defect in the development of the pleuroperitoneal fold arises from a disruption of a common signaling pathway that triggers mesenchymal differentiation of several different organ systems, leading to a global embryopathy with CDH being one of several manifestations.25 This has led to the hypothesis that the diaphragmatic defect and pulmonary hypoplasia seen in patients with CDH arise from a single “mesenchymal hit” rather than purely from a cause-effect relationship.7 Approximately 80% of BHs are left sided, and bilateral hernias occur rarely.7 A hernia sac may be present in up to 15% of cases.7
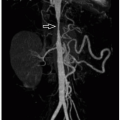
CDHs are frequently diagnosed on prenatal imaging. In general, patients with CDH have a survival rate of 70%, but rates are lower among patients with chromosomal and genetic syndromes, organ malformations, and severe isolated CDH.26 Survival rates are higher among patients diagnosed postnatally as compared with patients diagnosed prenatally, reflecting the fact that prenatally diagnosed patients generally have more severe disease.27 Fetal US and MRI can evaluate for severity of CDH and provide important prognostic information before birth. Parameters measured on fetal US include lung-to-head ratio and presence/absence of liver herniation. Parameters measured on fetal MRI include total lung volume and percent liver herniation. These parameters help assess the severity of disease and help predict mortality.26 Because of MRI’s superior contrast resolution, MRI parameters have proven more accurate in predicting survival than US parameters, and fetal MRI is therefore being more widely adopted (Fig. 13.11).28,29,30
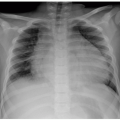
The first imaging study in a newborn with BH most frequently is a chest radiograph. The initial radiograph may show opacification of a hemithorax with contralateral mediastinal shift. If bowel loops have herniated into the thorax, follow-up radiographs will show gas-filled bowel in the thorax as the infant swallows air (Fig. 13.8). Abdominal radiographs may demonstrate a paucity of intra-abdominal bowel loops if a large amount of bowel has herniated into the thorax.31 If intra-abdominal organs have herniated into the thorax, opacity will persist on follow-up radiographs. Nasogastric tube (NGT) and umbilical venous catheter (UVC) position can be helpful in determining hernia contents and degree of mass effect (Fig. 13.8). As the NGT passes through the esophagus, it will deviate away from the side of the hernia because of mass effect. If the stomach has herniated into the chest, the tip of the NGT will be located within the thorax. In cases of liver herniation, UVCs will often have an altered position. The position of umbilical artery catheters can also be altered, but they are frequently less affected than UVCs and NGTs because of the aorta’s location within the retroperitoneum and posterior mediastinum.7 Identification of these findings on chest radiographs is essential for
confirmation of CDH, though specific findings on radiographs are not predictive of overall outcome.32 US may be helpful to confirm or characterize solid organ position prior to surgery (Fig. 13.12).
confirmation of CDH, though specific findings on radiographs are not predictive of overall outcome.32 US may be helpful to confirm or characterize solid organ position prior to surgery (Fig. 13.12).
 FIGURE 13.10 Newborn baby girl with a prenatal diagnosis of a right-sided Morgagni congenital diaphragmatic hernia who presented with severe perinatal respiratory distress. Frontal chest and abdomen radiograph shows complete opacification of the right hemithorax because of a complete liver (L) herniation causing shift of the heart (H) to the left and displacement of the nasogastric tube. There is bowel (B) in the right upper quadrant with a lack of a hepatic shadow.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|