Primary/idiopathic forms
Secondary forms
Acute exacerbation of IPF and NSIP
Bronchiectasis
Cryptogenic organizing pneumonia (COP)
Emphysema
Idiopathic pulmonary fibrosis (IPF)
Hematogenous metastases
Nonspecific interstitial pneumonia (NSIP)
Hydrostatic pulmonary edema
Infections
Lipoid pneumonia
Lymphangitic carcinomatosis (LC)
Nonspecific interstitial pneumonia (NSIP)
Organizing pneumonia (OP)
Silicosis and coal worker pneumoconiosis (CWP)
16.2 Radiological “Tools of the Trade”: HRCT
Patients with clinically significant DLD often have abnormalities detectable by radiological imaging of the chest. Unfortunately, plain chest radiographs rarely can make the diagnosis and may be misleadingly negative in up to 10 % of all patients with clinically significant interstitial lung disease. The current gold standard radiological technique for DLD is HRCT. This method has been widely accepted as the imaging standard of reference for assessing diffuse lung disease (Padley et al. 1991).
16.2.1 The HRCT Old Era
Before the introduction of modern volumetric CT, HRCT was achieved with 1-mm-thick sections obtained at 10 mm. HRCT images acquired at multiple selected levels show the representative areas of disease. The remarkable ability of HRCT to provide sufficient morphological detail of normal and abnormal lung parenchyma is based on well-done, high-quality examinations. Optimal visualization of the lung parenchyma is achieved by enhanced spatial resolution. The two most important factors in increasing spatial resolution are the use of thin collimation and reconstruction of the scan data with a high-spatial frequency algorithm. These two factors are essential for HRCT of the lung (Studler et al. 2005).
16.2.2 The HRCT New Era
Volumetric multidetector CT (MDCT) scan protocols have the advantage of great lung coverage and contiguous visualization of the lung parenchyma. From the volumetric high-resolution MDCT data sets with almost isotropic voxels, high-quality images in different, non-axial planes can be reconstructed. Several studies have already looked into the performance of volumetric high-resolution MDCT and demonstrated that MDCT is approximately equivalent to HRCT in some respects, but offers a huge amount of additional information which makes the assessment of diffuse lung disease much easier. Abnormalities are more easily identified and related to the underlying vascular, bronchial, and lobular anatomy.
16.2.2.1 Multiplanar Reconstruction (MPR)
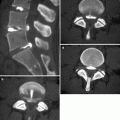
MPR provides diagnostic information comparable to that of axial HRCT while offering additional information in terms of lesion localization. Diseases that show uni- or bilateral and upper or lower lung prevalence benefit from visualization in coronal view (Fig. 16.1a). Finally, disorders that prevail in the parahilar regions or in the costophrenic angles are best depicted in the sagittal view. MPR may be also useful for easily viewing the deployment of fissures, indirect signs of volume loss in fibrosing lung diseases (Flohr et al. 2005). The sagittal reconstruction with bone window setting instantly shows the whole vertebral structure, useful in patients with possible associated bone lesions (Fig. 16.1b).
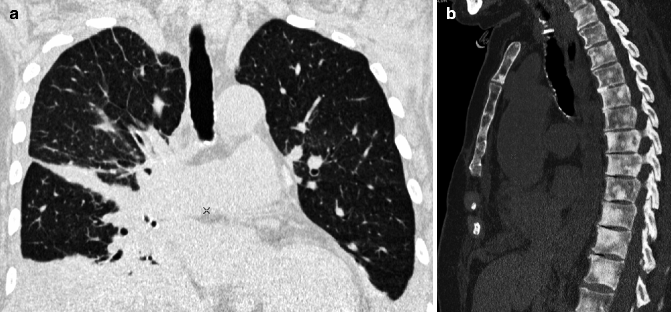

Fig. 16.1
Multiplanar reconstruction (MPR). Coronal view image easily reveals a unilateral and non-gravity-dependent reticular pattern (a). Sagittal reconstruction with bone window setting demonstrates diffuse multifocal involvement of bone in a single image (b)
16.2.2.2 Maximum Intensity Projection (MIP)
Consists of projecting the voxel with the highest attenuation value from every view throughout the volume onto a 2D image. The primary clinical application of MIP is to improve the detection of pulmonary nodules and assess their profusion. MIP also helps characterize the distribution of small nodules (Fig. 16.2a). In addition, MIP sections of variable thickness are excellent for assessing the size and location of vessels, including the pulmonary arteries and veins (Beigelman-Aubry et al. 2005).
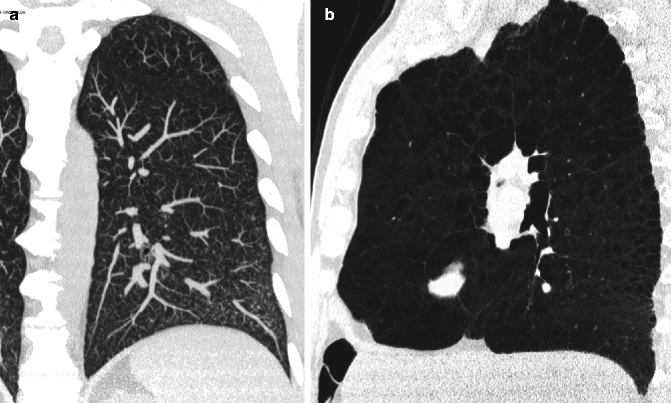

Fig. 16.2
MIP and minIP. MIP coronal image readily highlights diffuse small nodules and their profusion, thereby helping in the recognition of their characteristic distribution relative to the whole lung (a). On a sagittal minIP image, the high profusion of hyperlucent lesions in the left upper lobe becomes obvious (b)
16.2.2.3 Minimum Intensity Projection (minIP)
Consists of projecting the voxel with the lowest attenuation value from every view throughout the volume onto a 2D image. The subtle difference in density between the endobronchial (pure) air and the lung parenchyma, corresponding to a difference in attenuation of 50–150 HU, permits visualization of the bronchi below the subsegmental level. This technique is useful for improving the visualization of hyperlucent elements (bronchiectasis, emphysema, bullae, honeycombing) (Fig. 16.2b), and it is an optimal tool for the detection, localization, and quantification of ground-glass opacity and mosaic pattern.
16.2.2.4 Volume Rendering (VR)
VR techniques may be useful for studying a volume of lung in three dimensions from within or for inspecting its surface from outside. This is particularly useful for a concise look at the pulmonary surface and any abnormalities and for helping to make medical decisions (e.g., determining the site of a surgical biopsy) (Arakawa et al. 2004).
16.2.2.5 Pattern Approach to Diffuse Lung Diseases
A pattern-based HRCT approach to interstitial lung disease provides a “map” for the radiologist to navigate successfully the area of DLDs, especially so when used with the aid of the clinical patterns of presentation. A structured approach to interpretation of HRCT involves the following questions:
(a)
What is the dominant HRCT pattern?
(b)
Is there an upper versus lower zone or a central versus peripheral predominance?
(c)
Are there additional findings?
According to the literature and on the basis of the personal experience, the distinction of four main radiological patterns is suggested: reticular, nodular, alveolar, and cystic.
16.3 Reticular Pattern
16.3.1 Definition
On HRCT, a reticular pattern is present when a network of linear white lines is visible due to thickened septal, peribronchovascular, and subpleural interstitium. This finding is produced by a thickening of the structures of the lobular interstitium and often of the central interstitium as well. The interstitial thickening may be due to a variety of causes (fluid accumulation, cellular infiltration, fibrosis), and the pattern may vary accordingly (Andreu et al. 2004). The distribution of the lesions and other associated signs are often useful for diagnosis.
Morphologic characteristics of the septal thickening pattern allow the distinction of three possible subsets of the septal pattern: irregular (with distortion of architecture), smooth, and nodular (without distortion of architecture) (Table 16.2).
Table 16.2
Reticular pattern
Subsets | Diseases |
|---|---|
Irregular, fibrosing | Idiopathic pulmonary fibrosis (IPF) |
Nonspecific interstitial pneumonia (NSIP) | |
Smooth | Hydrostatic pulmonary edema |
Nodular | Lymphangitic carcinomatosis (LC) |
16.3.2 Reticular Pattern with Distortion (Irregular, Fibrosing)
In patients who have interstitial fibrosis, septal thickening visible on thin-section CT scans is irregular in appearance and associated with distortion of lung structures. This pattern is accompanied by other signs of fibrosis such as honeycombing, traction bronchiectasis, and bronchiolectasis, with vessels and bronchi following an irregular, corkscrew-like path (Fig. 16.3). These abnormalities result in variable parenchymal distortion and loss of volume. The prototype disease of fibrosing reticular pattern in elderly is idiopathic pulmonary fibrosis (IPF). Another possible aging-related fibrosing condition is nonspecific interstitial pneumonia (NSIP), primary or secondary (Table 16.2).
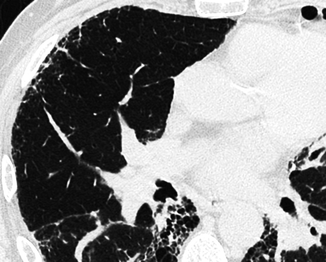

Fig. 16.3
Reticular pattern, subset irregular (fibrosing). There is evidence of peripheral irregular interstitial lines and evidence of ectatic bronchiole with corkscrew-like path. These signs bear witness to a fibrotic disease, causing retraction and remodeling of pulmonary structures
16.3.2.1 Idiopathic Pulmonary Fibrosis (IPF)
IPF is an aging-related DLD; in fact, studies have confirmed that IPF is predominantly a disease of elderly individuals, with a mean age at presentation of 60–65 years. IPF is also the most common entity of DLD and is the most frequent of the idiopathic interstitial pneumonias. With a median survival time ranging from 2 to 4 years, IPF has a substantially poorer prognosis than other interstitial pneumonias. IPF-related mortality seems to be higher in the elderly, although studies related to this concern are scanty. The effect of age at diagnosis on survival was explored by King et al. a few years ago (King et al. 2001). In the study, the median survival time was significantly shorter in older individuals (60–70 years of age) than younger ones (<50 years of age). These findings indicate that IPF is not only an age-related disease but also that aging affects the survival rate.
The HRCT pattern reflects the pathologic aspect called usual interstitial pneumonia (UIP) featuring the presence of retraction and remodeling (fibrosing pattern). The typical CT findings consist of patchy peripheral areas of irregular reticulation prevalent in the middle-upper zones. The patchy areas result from a combination of intralobular lines and irregular septal thickening, but the lobular architecture is often so distorted that these may be impossible to recognize. In the basal and subpleural zone, the other signs of fibrosis prevail and in particular honeycombing. Honeycombing is a common and a key sign for diagnosis of IPF. It is manifested on HRCT as clustered cystic airspaces, typically of comparable diameters on the order of 3–10 mm but occasionally as large as 2.5 cm. It is usually subpleural and is characterized by well-defined walls (Hansell et al. 2008). Often dilated and distorted bronchioles (traction bronchiolectasis) and bronchi (traction bronchiectasis) coexist. Rugged pleural surfaces (interface sign) are very frequent. Some GGO is possible but it is less extensive than the reticulation. Characteristically, the patchy areas of fibrosis are intermingled and sharply marginated with areas of normal parenchyma (Fig. 16.4a) (Mueller-Mang et al. 2007; Silva and Müller 2009). Mild mediastinal lymph node enlargement is evident on CT in approximately 70 % of patients with IPF. The lymphadenopathy usually involves only one or two nodal stations, and the nodes usually measure less than 15 mm in short axis diameter (Fig. 16.4b). The likelihood of lymphadenopathy increases with the extent of parenchymal involvement and decreases in the presence of recent steroid treatment (Souza et al. 2006).
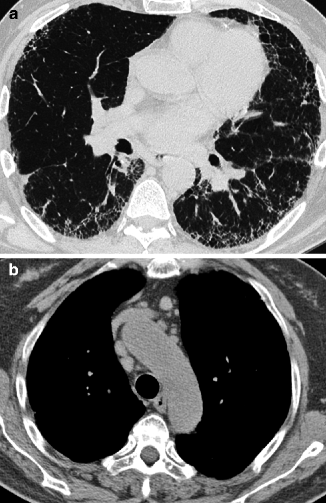

Fig. 16.4
Idiopathic pulmonary fibrosis (IPF). Basal and peripheral areas of irregular reticulation and honeycombing with prominent signs of architectural distortion (a). Mediastinal lymph nodes enlargement (b)
The HRCT is an essential component of the diagnostic pathway in IPF, as indicated in the recent new official multidisciplinary statement. The evidence-based guidelines suggest that the diagnosis of IPF requires (1) exclusion of other known causes of DLD (e.g., domestic and occupational environmental exposures, connective tissue disease, and drug toxicity), (2) the presence of a UIP pattern on HRCT in patients not subjected to surgical lung biopsy, and (3) specific combinations of HRCT and surgical lung biopsy pattern in patients subjected to surgical lung biopsy (Raghu et al. 2011). The accuracy of diagnosis of IPF increases with clinical, radiological, and histopathologic correlation and can be accomplished with a multidisciplinary discussion among experienced clinical experts in the field of ILDs (Flaherty et al. 2004).
16.3.2.2 Idiopathic Nonspecific Interstitial Pneumonia (NSIP)
The mean age at onset is 40–50 years, although elderly patients may also be affected. Idiopathic NSIP is one of the idiopathic interstitial pneumonias which in certain aspects is similar to UIP, although it often has a more favorable prognosis. As the name suggests, the clinical findings in NSIP are often nonspecific and the patients have a gradual onset of symptoms.
The key radiological finding for diagnosis of NSIP is the presence of irregular reticulation associated with homogeneous areas of GGO (Fig. 16.5a). The percentage of each likely depends on the proportions of inflammation and fibrosis within the lung, and some authors have even attempted to identify definite subgroups based on the extent of reticulation and traction bronchiectasis (Johkoh et al. 2002). Traction bronchiectasis and bronchiolectasis are characteristic. Honeycombing should be absent or minimal, unlike IPF (Silva and Müller 2009; Kligerman et al. 2009).
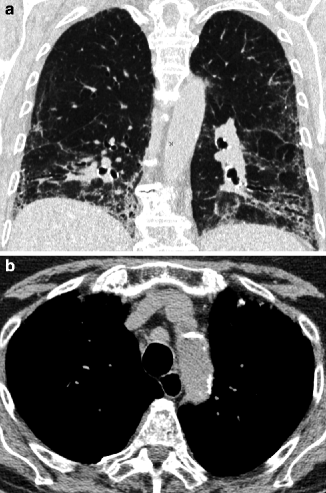

Fig. 16.5
Nonspecific interstitial pneumonia (NSIP). Coronal view shows loss of volume of the bases with lower displacement of the fissures. Areas of GGO with irregular reticulation and traction bronchiectasis coexist (a). Mediastinal window displays the dilated esophagus behind the trachea and lymph node enlargement (b)
16.3.2.3 Secondary NSIP (Collagen Vascular Diseases, Drug Toxicity)
NSIP associated with collagen vascular disease (scleroderma, polymyositis and dermatomyositis, Sjögren syndrome, and rheumatoid arthritis) is more prevalent in women than in men. Given this association, it is important to look for additional abnormalities that may aid in diagnosis. Findings that can suggest an underlying collagen vascular disease include esophageal abnormalities (Fig. 16.5b) and pleural or pericardial effusions (Kim et al. 2002).
NSIP may be also associated with toxic effects of drugs. Several adverse reactions to therapeutic drugs may present with aspects indistinguishable from the idiopathic NSIP. Consequently, the diagnosis of the underlying disorder should be formulated on clinical grounds (Rossi et al. 2000; Camus et al. 2004).
16.3.3 Reticular Pattern Without Distortion (Smooth)
The three interstitial compartments (perilobular, peribronchovascular, and subpleural) are thickened without distortion. The lobular architecture is preserved, only it is more recognizable than in the normal lung, with an exaggerated appearance at times (Fig. 16.6). The prototype disease of this subset is interstitial hydrostatic edema (Table 16.2).
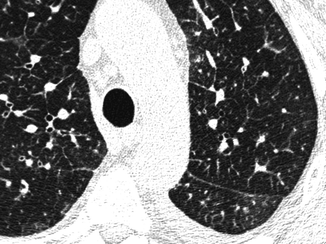

Fig. 16.6
Reticular pattern, smooth. Some septa and a thickened fissure are visible together with a bronchial cuffing. All these signs present smooth profiles without focal abnormalities
16.3.3.1 Hydrostatic Pulmonary Edema
This type of pulmonary edema is a frequent cause of admission to the hospital in particular in elderly patients. The diagnosis of hydrostatic pulmonary edema is usually based on clinical information, conventional chest radiograph findings, and response to treatment and does not require HRCT. However, recognizing the appearance of hydrostatic pulmonary edema on HRCT can be important, as the edema can mimic other diseases or can occur as an unsuspected finding in patients having HRCT for other indications, and a misdiagnosis may lead to an unnecessary lung biopsy.
On HRCT scans, smooth thicker bronchial walls are seen, together with an increase in the diameter of the central and peripheral pulmonary vessels. Another aspect of interstitial edema is the presence of GGO (Fig. 16.7a). These areas are predominantly found in the lower potions of the lungs due to the gravitational effect. Often also present are other associated radiological findings that reinforce a diagnosis of pulmonary edema, particularly heart enlargement and uni- or bilateral pleural effusion (Fig. 16.7b) (Ribeiro et al. 2006). When the flow of lymph to the systemic veins decreases, an enlargement of mediastinal lymph nodes due to fluid stagnation may occur (Chabbert et al. 2003).
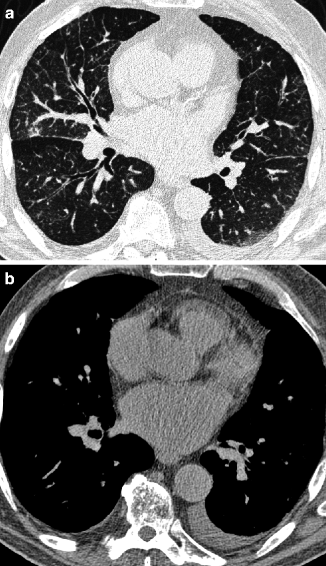

Fig. 16.7
Hydrostatic pulmonary edema. The three interstitial compartments (perilobular, peribronchovascular, and subpleural) are thickened with smooth profiles. GGO and increase in the diameter of pulmonary vessels are also present (a). Mediastinal window shows unilateral small pleural effusion and enlarged left atrium (b)
16.3.4 Reticular Pattern Without Distortion (Nodular)
The interstitial compartments are thickened in nodular form, testifying to the existence of locally growing cells or extracellular deposits within the interstitial boundaries. Being interstitial, these nodules are dense with well-defined margins, embedded as they are inside thickened interlobular septa and interstitial lines with an overall beaded appearance (Fig. 16.8). The prototype disease of this subset is pulmonary lymphangitic carcinomatosis (Table 16.2).
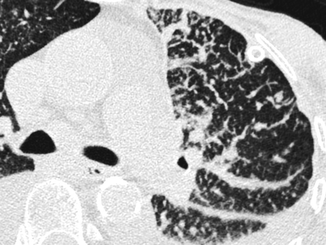

Fig. 16.8
Reticular pattern, subset nodular. Diffuse thickening of many interlobular septa. Inside the septa, some dense nodules with well-defined margins and different sizes are visible, some of them very small. Nodular thickening is also visible along the subpleural space
16.3.4.1 Lymphangitic Carcinomatosis (LC)
LC usually affects adult patients, but it is a growing cause of DLDs in the elderly because it is related to increased life expectancy. It is defined as diffuse infiltration and obstruction of the pulmonary lymphatic vessels by malignant cells. Various neoplasms can cause pulmonary LC, the most common being adenocarcinoma of the lung, breast, stomach, colon, and prostrate, in order of frequency.
HRCT images show beaded thickening of the bronchovascular bundles, interlobular septum, and subpleural interstitium. Nodules result from focal growth of cells within the lymphatics and local extensions into the parenchyma. The lesions of lymphangitic carcinomatosis are typically patchy, often unilateral, and not gravity dependent (Fig. 16.9a). They may coexist with unilateral or bilateral random nodules due to hematogenous metastases, lymphadenopathy (30–50 %), or pleural effusion (30–50 %). Dedicated CT window settings may demonstrate metastatic lesions elsewhere (Fig. 16.9b).
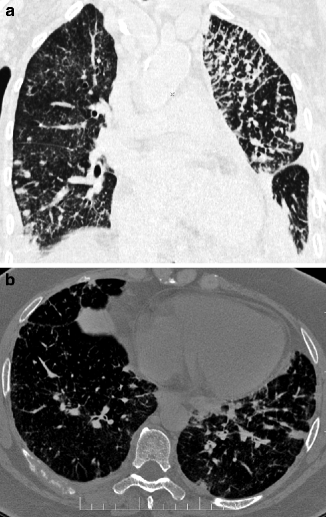

Fig. 16.9
Lymphangitic carcinomatosis. The coronal view shows a unilateral reticular pattern with nodular subset and non-gravity distribution. In the right lower lobe, some random nodules due to hematogenous metastases are also visible (a). Bone window setting shows a lytic involvement of a right posterior rib (b)
16.4 Nodular Pattern
16.4.1 Definition
Nodular pattern is defined by the presence of multiple white, roundish, pulmonary opacities ranging in diameter from 2 to 10 mm. The nodular pattern may be due to a variety of diseases. Some arise directly in the lung or arrive via the bloodstream in the small vessels where they develop concentrically, or via the bronchi, for example, when a reaction to an inhaled substance develops a small bronchus.
The topographical distribution of the nodules is a helpful aid to distinguish subsets of nodular disorders (Gruden et al. 1999; Raoof et al. 2006). Lesions that spread hematogenously are visible everywhere, so they may be seen in the core but also in the periphery (random), sometimes in connection with blood vessels. The diseases that grow along the lymphatics are more likely present in the periphery of the lobules and in particular along the fissures (lymphatic). The inhaled diseases show nodules close to the bronchiole in the center of lobules (centrilobular) (Table 16.3).
Table 16.3
Nodular pattern
Subsets | Diseases |
|---|---|
Random | Hematogenous metastases |
Miliary TB | |
Lymphatic | Silicosis and coal worker pneumoconiosis (CWP) |
Centrilobular | Hypersensitivity pneumonitis (HP), subacute |
16.4.2 Random Nodules
Random nodules are homogeneously distributed in the lungs, touching the pleural surfaces but without a consistent relationship with them (“indifferent to the pleura”) (Maffessanti and Dalpiaz 2006). At times they may be in contact with the extremities of vascular structures (feeding-vessel sign). More frequently they have high density and well-defined margins (Fig. 16.10); in specific conditions they may show cavitation. Diseases in the nodular pattern, subset random, are hematogenous metastases and miliary TB (Table 16.3).
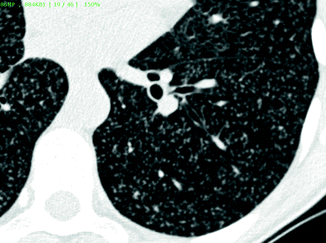

Fig. 16.10
Random nodules. Innumerable tiny nodules are scattered throughout the lung. They are dense and uniform in size. The nodules are “indifferent for the pleura.” At times, they are in contact with the extremities of vascular structures (feeding-vessel sign)
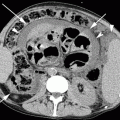
16.4.2.1 Hematogenous Metastases
Secondary neoplastic involvement of the lungs is extremely common. Large autopsy series of patients with extrathoracic malignancies reveal pulmonary metastases in 20–54 % of patients (Libshitz and North 1982).
Typical HRCT findings of hematogenous metastases include multiple random nodules, at times in contact with the extremities of vascular structures (feeding-vessel sign). The round nodules are usually dense and well defined with variable size. The nonuniform diameter of the lesions may be related to subsequent episodes of metastatic spread (Fig. 16.11a) (Hirakata et al. 1993). Nodules with poorly defined margins can be identified in 16–30 % of cases; these may reflect lepidic growth of tumor. The presence of an intranodular hyperlucency gives the lesion a characteristic appearance known as the “Cheerios pattern” (after the American breakfast cereal). Cavitation is indicative of a squamous-cell carcinoma of the head or neck, uterine cervix, and bladder or, less frequently, of an adenocarcinoma (especially gastrointestinal) or sarcoma (Seo et al. 2001). A basilar predominance is typically noted due to preferential blood flow to the lung bases. When limited in number, metastatic nodules may be seen primarily in the lung periphery. In patients who have innumerable metastases, uniform distribution throughout the lung is common. Possible associated signs include mediastinal adenopathy (Fig. 16.11b) and lymphangitic carcinomatosis.
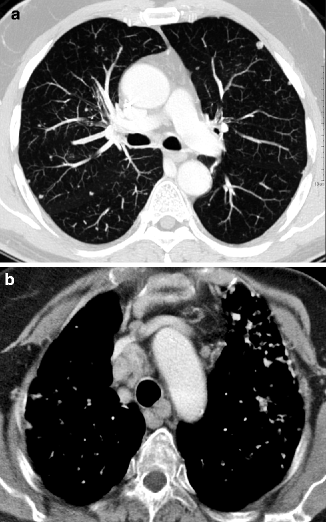

Fig. 16.11
Hematogenous metastases. On the bases, scattered nodules with random distribution and variable size are visible. Some of them are feeding-vessels (a). The contrast-enhanced CT image displays several enlarged mediastinal lymph nodes (b)
16.4.2.2 Miliary Tuberculosis
Miliary TB is more common among men and the elderly; age is one of the possible risk factors together with socioeconomic status (malnutrition and poverty), drug and alcohol abuse (probable negative action against the immune system), race and genetic factors (the disease is more common and severe among African Americans than among whites), associated diseases (silicosis, diabetes, chronic renal failure), and immunosuppression (HIV, steroid treatment, transplant patients) (Tobin 2004).
The term miliary tuberculosis encompasses all forms of progressive tuberculosis (TB) with hematogenous seeding. Indeed radiologically the nodules present a random distribution. At times, they are in contact with the extremities of vascular structures (feeding-vessel sign). The term miliary is also indicative of the size of the nodules (very small, 2–3 mm). They are dense and uniform in size, either sharply or poorly defined (Fig. 16.12a). The nodules are distributed uniformly throughout the lungs often without cephalocaudal or central-to-peripheral preference (Cancellieri et al. 2010).
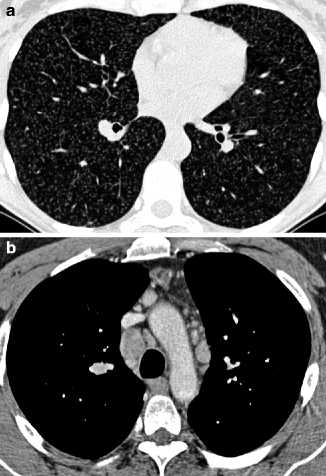

Fig. 16.12
Miliary tuberculosis. Innumerable nodules of miliary size are scattered throughout both lungs with random distribution (“indifferent to the pleura”). The nodules are dense and uniform in size (a). The contrast-enhanced CT image displays mediastinal enlarged lymph nodes, some of them hypodense due to colliquative phenomena (b)
The diagnosis may be suggested by the presence of associated findings and in particular consolidation, signs of bronchogenic spread of the disease with tree-in-bud pattern, and lymphadenopathy. Necrosis is the radiological and pathological marker for infection, visible as cavitated lesions (nodules, consolidations, and lymphadenopathy) (Fig. 16.12b) (Burrill et al. 2007). Signs from previous tuberculosis are seen in 50 % of patients and aid in the differential diagnosis. Such changes often occur in the upper lobes as fibrotic bands with traction bronchiectasis, apical calcified nodules, and areas of oligemia due to previous bronchiolitis obliterans (Burrill et al. 2007; Jeong and Lee 2008).
16.4.3 Lymphatic Nodules
The lymphatic nodules tend to be prevalent in the perilobular and subpleural interstitium and are therefore profuse along the costal margins and the fissures (“avid of pleura”) (Fig. 16.13) (Maffessanti and Dalpiaz 2006). They are more common in diseases which spread along the lymphatics, and may therefore be found within the lobule, but also along the vessels and bronchi (beaded appearance). The nodules have well-defined margins as well as high and uniform opacity. The spatial arrangement of the lesions tends to be patchy, interspersed with areas of normal parenchyma. The prototype diseases with nodular pattern, subset lymphatic, are silicosis and coal worker pneumoconiosis (Table 16.3).
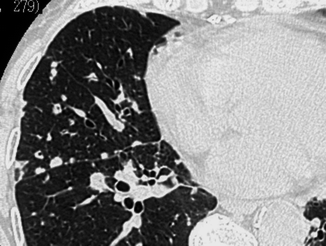

Fig. 16.13
Lymphatic nodules. The nodules are preferentially located in the perilobular and subpleural interstitium, more visible along the fissure (“avid of pleura”)
16.4.3.1 Silicosis and Coal Worker Pneumoconiosis (CWP)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree