(1)
Department of Nuclear Medicine, Kuwait University, Safat, Kuwait
9.1 The Esophagus
9.2 The Stomach
9.2.3 Duodenogastric Reflux
9.3 The Intestines
9.3.1 The Small Intestine
9.3.2 The Colon
9.4 Salivary Gland
9.5 Ascites
9.6.3 Gastric Emptying Study
9.6.8 Salivary Gland Imaging
9.6.9 Imaging of Appendicitis
9.6.10 Nonimaging Procedures
Abstract
The digestive system consists of the gastrointestinal tract, hepatobiliary system, pancreas, and salivary glands. Nuclear medicine is concerned with evaluation of normal and abnormal functions particularly of the gastrointestinal tract and the hepatobiliary system.
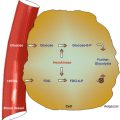
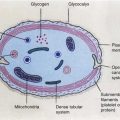
The digestive system consists of the gastrointestinal tract, hepatobiliary system (Fig. 9.1), pancreas, and salivary glands. Nuclear medicine is concerned with evaluation of normal and abnormal functions particularly of the gastrointestinal tract and the hepatobiliary system.
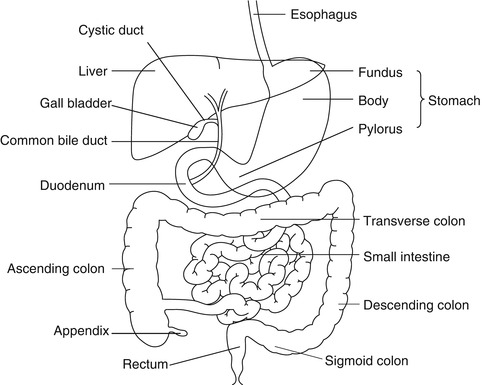

Fig. 9.1
Diagram of the relevant parts of the digestive system
9.1 The Esophagus
9.1.1 Anatomical and Physiological Considerations
The human esophagus is a hollow muscular tube that connects the pharynx to the stomach. The upper third consists of striated muscle, the lower third is composed of smooth muscle, and the middle third is a mixture of the two. Functionally, the esophagus has three components: the upper esophageal sphincter (UES), the esophageal body, and the lower esophageal sphincter (LES). These components act to keep the esophagus empty so that swallowed food or liquid is propelled from the pharynx to the stomach and also prevent retrograde movement of gastric or esophageal content.
Upper esophageal sphincter is defined as a high-pressure zone, 2–7 cm long, separating the pharynx from the body of the esophagus. It consists of opening and closing muscles. The esophageal body extends from the UES to the LES and measures 18–24 cm [1]. The lower esophageal sphincter is a high-pressure zone measuring 2–4 cm in length located between the esophageal body and the stomach. At rest the sphincter is tonically contracted with a normal pressure ranging from 10 to 45 mmHg. On swallowing the LES relaxes and its pressure approaches that of the stomach. The LES remains relaxed until the bolus reaches the end of the esophagus.
Swallowing initiates a progressive series of coordinated propulsive contractions throughout both the striated and the smooth muscle portions of the esophageal body. This form of esophageal motor activity is referred to as primary peristalsis. Intraluminal distention of the esophageal body results in a peristaltic wave at or proximal to the site of distention. This wave is termed secondary peristalsis and serves to clear the esophagus from contents that have not been cleared by primary peristalsis or refluxed gastric contents. Primary and secondary peristaltic waves have similar amplitudes and travel at a velocity of 3–5 cm/s.
Deglutitory inhibition is a unique physiological phenomenon whereby repetitive swallowing inhibits all esophageal body activity while the LES is relaxed. A normal peristaltic contraction will follow the last swallow of such a series and clear the esophagus completely [2].
9.1.2 Esophageal Motility Disorders
Esophageal motility disorders result from sphincter dysfunction or abnormal peristalsis in the body of the esophagus or both. The diagnosis and treatment of motility disorders rest on an understanding of the functional anatomy of the upper esophageal sphincter, esophageal body, and lower esophageal sphincter.
9.1.2.1 Disorders of the UES and Cervical Esophagus
Motor disorders affecting the proximal part of the esophagus result from either neurological abnormalities affecting the extrinsic innervation of the proximal esophagus or skeletal muscle or neuromuscular disorders. These include:
Neurological diseases
Cerebrovascular accident
Parkinsonism
Amyotrophic lateral sclerosis
Cranial nerve palsy
Skeletal muscular disorders
Dermatomyositis
Polymyositis
Muscular dystrophy
Cricopharyngeus dysfunction
Others
Myasthenia gravis
Amyloidosis
Because of the difficulty of transferring food bolus from the hypopharynx into the esophageal body across the UES, most patients experience choking or regurgitation of liquids and/or solids. Videofluoroscopy is the best diagnostic modality for diagnosing oropharyngeal dysphagia. Scintigraphy is of limited value.
9.1.2.2 Disorders of Distal Esophagus and LES
Disorders of the distal esophageal body (smooth muscle) and LES can be broadly classified into hypermotility and hypomotility disorders, although overlap is not uncommon.
9.1.2.2.1 Hypermotility Disorders
The hypermotility (spastic) disorders are characterized by high-amplitude, prolonged, or repetitive contractions. They include achalasia and several other conditions, particularly the diffuse esophageal spasm (Table 9.1).
Table 9.1
Manometric features of distal esophageal disorders
Disorder | Features |
|---|---|
Achalasia | Absent peristalsis in esophageal body |
Incomplete LES relaxation | |
Increased LES pressure | |
Diffuse esophageal spasm | Simultaneous contractions (>10 % of wet swallows) |
Intermittent normal peristalsis | |
Repetitive contracts | |
Prolonged duration of contractions | |
High amplitude of contractions | |
Incomplete LES relaxation | |
Nutcracker esophagus | Normal progression of peristalsis |
Mean contractile amplitude 180 mmHg | |
Prolonged duration | |
Normal LES pressure | |
Hypertensive LES | Mean LES pressure 45 mmHg |
Normal LES relaxation | |
Normal progression of peristalsis |
Achalasia results from the degeneration of the inhibitory myenteric neurons in the body and the LES region. This leads to a hypertensive LES which relaxes poorly and also causes aperistalsis in the body of the esophagus. Patients usually present with dysphagia to liquids and solids.
Several other spastic disorders have been characterized in patients with noncardiac chest pain (Table 9.1). They all share a similar clinical presentation. Diffuse esophageal spasm (DES) is the most severe form. It is less common than achalasia. Some patients with DES progress to achalasia.
9.1.2.2.2 Hypomotility Disorders
A number of systemic conditions are associated with esophageal hypomotility including scleroderma, diabetes mellitus, and amyloidosis. These disorders are characterized by low or absent contractions. The most clinically relevant condition is scleroderma.
Scleroderma (progressive systemic sclerosis) is a multisystem connective tissue disorder that affects the skin and internal organs, especially the vascular system, gastrointestinal tract, lungs, heart, and kidneys. It is more common in women than in men and appears at any age under 50 years. Esophageal involvement occurs in 70–80 % of patients. Histopathological findings include smooth muscle atrophy and fibrosis. The end result is impaired muscle contractions in the distal esophagus and incompetence of the LES. Therefore, 50 % of patients with scleroderma-associated dysmotility complain of heartburn and/or dysphagia due to gastroesophageal reflux.
9.1.2.3 Gastroesophageal Reflux Disease
Gastroesophageal reflux disease (GERD) involves the reflux of chyme from the stomach to the esophagus. The LES may relax spontaneously and transiently 1–2 h after the patient has eaten, allowing gastric contents to regurgitate into the esophagus. The acid is normally neutralized and cleared by peristalsis from the esophagus within 3 min, and the tone of the sphincter is restored. When the reflux does not cause symptoms, it is known as physiological, but in some individuals it may cause a spectrum of inflammatory responses in the esophagus. GERD is the most prevalent condition originating from the gastrointestinal tract. It is estimated that 20 % of the Western adult population suffer from heartburn more than three times a month [3]. It is particularly important in the pediatric age-group. GERD is also common among pregnant women, especially during the third trimester. The typical symptom of GERD is heartburn. However, a number of atypical symptoms include noncardiac chest pain, hoarseness, and asthma.
Most children affected with gastroesophageal reflux are between 6 months and 2 years old; they suffer from poor weight gain, vomiting, aspiration, choking, asthmatic episodes, stridor, apnea, and failure to thrive. A small amount of physiological reflux occurs in infants and resolves spontaneously by 8 months of age. Scintigraphy is useful in the diagnosis and is physiological, easily performed, well tolerated by the patient, quantitative, and involves a low radiation dose to the child.
Causes of GERD can be categorized as follows: (a) decreased pressure of LES, (b) transient increase in intra-abdominal pressure, and (c) short intra-abdominal esophageal segment. Mechanisms involved are summarized in Table 9.2. Esophageal body peristalsis plays an important role in clearing refluxed acid in both the upright and the supine position. Defective primary or secondary peristalsis leads to incomplete clearance of acid. Furthermore, salivary HCO3 usually neutralizes acid that remains in contact with the esophageal mucosa. Thus, impaired salivation may contribute to mucosal injury [4].
Table 9.2
Mechanisms of gastroesophageal reflux disease
Mechanism | Causes |
|---|---|
Anti-reflux barrier | Transient LES relaxation |
Incompetent LES | |
Sliding hiatus hernia | |
Esophageal clearance | Impaired peristalsis |
Decreased salivary output | |
Refluxate composition | Acid |
Pepsin | |
Bile salts | |
Pancreatic enzymes | |
Gastric factors | Delayed gastric emptying |
Acid hypersecretion | |
Helicobacter pylori | |
Defective esophageal mucosal protection | Lack of HCO3 secretion |
Lack of mucus secretion |
Delayed gastric emptying is documented in 6–30 % of patients with GERD. Theoretically, gastric stasis can contribute to GERD. However, the relative importance of delayed gastric emptying is not well established [4]. Helicobacter pylori has recently been implicated as having a potential role in the pathogenesis of GERD [5]. H. pylori may secrete proinflammatory substances that can damage esophageal mucosa and sensitize vagal afferent nerves or lead to the reduction of LES tone. In contrast, there are data suggesting a protective role for H. pylori against GERD [6].
9.2 The Stomach
9.2.1 Anatomical and Physiological Considerations
9.2.1.1 Anatomical Features
The stomach is a storage sac located between the esophagus and duodenum (Fig. 9.1). The proximal stomach consists of the cardia, fundus, and body. The antrum forms the distal stomach and is separated from the duodenum by the pyloric ring. The wall structure of the stomach is similar to that of the rest of the gastrointestinal tract consisting of the mucosa, submucosa, muscularis propria, and serosa. The muscle layer in the antrum is modified to aid the mixing of food. The pyloric ring regulates the emptying of the stomach.
9.2.1.2 Overall Functions
Besides storage, the stomach has a number of exocrine, paracrine, and endocrine functions. The exocrine secretions consist of HCI and pepsin produced by the mucosal parietal cells and chief cells, respectively. These cells are located in the fundus and body of the stomach. Most cells within the lamina propria and submucosa are responsible for the main paracrine function, namely, the release of histamine which in turn stimulates the parietal cells to secrete acid. The antrum secretes the hormone gastrin which enhances gastric emptying and acid secretion. The intrinsic factor (IF) is a glycoprotein secreted by parietal cells. It binds to vitamin B12. The IF-B12 complex in turn binds to specific receptors on the terminal ileal epithelium. Without IF, B12 cannot be absorbed and pernicious anemia develops. Usually failure to secrete IF results from gastric atrophy which causes the destruction of parietal cells.
9.2.1.3 Gastric Motor Physiology
The motor activity of the stomach serves two main functions: (a) to act as a reservoir for ingested meal and ensure timed delivery of food particles to the duodenum at a rate compatible with optimal digestion and (b) to disperse solids into small particles and to mix them with gastric juice. The functions are accomplished by the coordinated activity of three functionally distinct parts of the stomach: (a) the proximal stomach, including the fundus and proximal body; (b) the distal stomach, including distal body and antrum; and (c) the pylorus, as part of the pyloroduodenal unit.
The proximal stomach has three muscle layers, longitudinal, circular, and oblique. The distal stomach is comprised of two muscle layers: the longitudinal and circular. The pyloric sphincter functions, in coordination with the duodenum, as a sieve allowing particles l mm or smaller to pass into the duodenum in 2–4-ml aliquots with each gastric peristalsis [7]. Emptying of inert liquids such as 0.9 % saline follows first-order kinetics; i.e., the volume of liquid emptied into the duodenum in a given time is a constant fraction of the volume that remains in the stomach (Fig. 9.2) [8]. Emptying of digestible solid particles is characterized by a lag phase and a linear phase (Fig. 9.3) [8]. However, the components, caloric density, viscosity, osmolarity, and volume of any specific meal will influence gastric emptying rates.
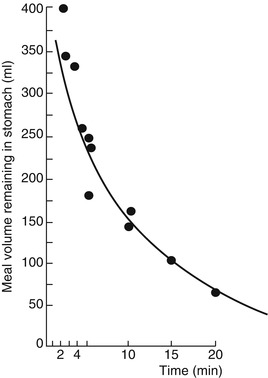
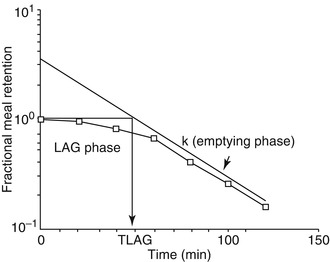

Fig. 9.2
Gastric emptying of 0.9 % normal saline follows first-order kinetics (From Brener et al. [8])

Fig. 9.3
Emptying of digestible solid particles has a lag phase and a linear phase. The curve represents modified power exponential function (From Siegel et al. [9])
9.2.2 Disorders of Gastric Emptying
Conditions that cause abnormal gastric emptying can be divided into two groups: disorders associated with delayed emptying and disorders associated with dumping (Table 9.3). Diabetes is one of the most common causes of delayed gastric emptying in clinical practice. Most afflicted patients have had type I diabetes for more than 10 years, complicated by autonomic and peripheral neuropathy. Delayed emptying of both solids and liquids is attributed to the dysfunction of the proximal and distal stomach, as well as to increased pyloric resistance [10].
Table 9.3
Causes of gastric dysmotility
Condition | Causes |
|---|---|
Delayed gastric emptying | Mechanical obstruction |
Gastric outlet obstruction (e.g., tumor, peptic ulcer) | |
Small intestinal obstruction | |
Decreased gastric motility | |
Postsurgical gastroparesis (vagotomy, Roux-en-Y, fundoplication, etc.) | |
Endocrine disorders (DM, hypothyroidism, Addison’s disease, hyper- or hypoparathyroidism) | |
Drugs (narcotics, anticholinergics, calcium channel blockers) | |
Connective tissue diseases (e.g., scleroderma, SLE) | |
Muscular disorders (myotonic dystrophy, dermatomyositis) | |
Paraneoplastic | |
Post-viral | |
Neurological disorders (migraine, CVA, Parkinson, dysautonomia) | |
Intestinal pseudo-obstruction | |
Idiopathic gastroparesis | |
Others (anorexia nervosa, uremia, ischemic gastroparesis, pregnancy) | |
Rapid gastric emptying (dumping) | Duodenal ulcer disease (including ZE syndrome) |
Vagotomy | |
Antrectomy | |
Idiopathic |
Idiopathic gastroparesis is also encountered frequently in patients with bloating, early satiety, and nausea. The exact cause is unclear but it may be related to post-viral gastroenteritis [11]. Delayed gastric emptying is a known and frequent complication of gastric surgery. For instance, vagotomy delays emptying of solids but promotes liquid emptying. Similarly antrectomy can cause rapid emptying of undigested solids and liquids due to the loss of mixing function and loss of pyloric resistance.
9.2.3 Duodenogastric Reflux
Duodenogastric reflux (DGR) has been suggested to occur in normal individuals in both fasting and postprandial periods [12, 13], although others have suggested that it does not occur physiologically [14]. The amount of refluxed bile in normal subjects is reported to be small and clears rapidly from the stomach [15]. This feature helps separate normal from abnormal subjects in the fasting state.
Pathologically, DGR has been associated with many conditions (Table 9.4), including postgastrectomy/vagotomy, gastric and duodenal ulcers, cholecystitis, and gastritis [16]. In some of these conditions, such as duodenal ulcer, duodenal hematoma, and cholecystitis, duodenal irritation is probably the underlying mechanism. Other causes may irritate the duodenal mucosa by the adjacent pancreatic inflammation. It was also proposed that deficiency of pancreatic secretions can explain DGR since volume, the alkaline pH, and the physiological components of the pancreatic secretions may be important for maintaining the outward flow of gastric contents [17].
Table 9.4
Causes of duodenogastric reflux
Causes | |
|---|---|
Duodenal ulcer | Gastritis |
Acute cholecystitis | Chronic cholecystitis |
Enteritis | Pancreatitis |
Gastric carcinoma | Surgery |
Post-traumatic stress ulceration | Gastric surgery/vagotomy |
Duodenal hematoma | Cholecystectomy |
Erosive esophagitis | Gallstone dyspepsia |
Physiological/unknown cause | |
9.3 The Intestines
9.3.1 The Small Intestine
9.3.1.1 Anatomical and Histological Considerations
The small intestine is a hollow muscular cylinder that measures 5–6 m in length. It consists of three regions: duodenum, jejunum, and ileum. The intestinal wall is made up of the mucosa, the submucosa, the muscularis, and the serosa. The small intestinal mucosa is fashioned into villi and crypts to increase the surface area and enhance the absorptive function of the small bowel. The mucosa of the villi consists of absorptive columnar epithelial cells (enterocytes) and mucus-secreting goblet cells. Within the crypts the most common cell type is the undifferentiated crypt cell which secretes chloride and water into the lumen. The crypt also contains pluripotent stem cells. Furthermore, the small bowel harbors the enteroendocrine cells which secrete a number of hormones including secretin, cholecystokinin, gastrin, gastric inhibitory peptide, motilin, glucagon, vasoactive intestinal peptide, somatostatin, and others. These hormones play an important role in gastrointestinal motility. Finally, the intestinal mucosa and lamina propria contain the largest lymphoid organ in man: the gut-associated lymphoid tissue (GALT) [18].
The submucosa consists of connective tissue, lymphocytes, plasma cells, macrophages, mast cells, fibroblasts, eosinophils, nerve fibers, ganglion cells (Meissner’s plexus), blood vessels, and lymphatics.
The muscularis is made of inner circular and outer longitudinal muscle fibers. Between these two layers of smooth muscle lies the myenteric plexus, the network of intramural neurons that is essential for all coordinated and organized motor activity. The extrinsic (autonomic) nerves affect the gastrointestinal motility by means of these enteric nerves.
9.3.1.2 Functional Considerations
Most of the digestion and absorption of nutrients takes place in the small bowel. Moreover, the motor function of the small bowel ensures the mixing of chyme with digestive enzymes and the propulsion of chyme toward the colon. Also, the small bowel plays an important role as a first line of defense against pathogenic microorganisms and harmful food antigens.
Absorption refers to the process of transporting molecules through the epithelial lining of the gastrointestinal tract into the blood or lymph. Water, electrolytes, monosaccharides, amino acids, small peptides, glycerol, fatty acid, vitamins, and minerals are all absorbed via a number of mechanisms including passive diffusion, facilitated diffusion, active transport, and endocytosis. Although absorption takes place along the entire length of the small intestine, the mucosa in certain regions selectively absorbs specific molecules. For instance, iron is primarily absorbed in the duodenum and proximal jejunum, whereas the terminal ileal mucosa has specific receptors for binding and absorbing vitamin B12 and bile salts.
Under physiological conditions the small bowel exhibits two main motor patterns. During the fed state, and as a result of contact with nutrients, a number of neuronal and hormonal signals are elicited including afferent vagal stimulation and the release of cholecystokinin which mediate segmentation and peristalsis. Segmentation is the most frequent movement in the small bowel and is characterized by closely spaced contractions of the circular muscle layer. These contractions divide the small intestine into short neighboring segments. Segmentation helps mix chyme with digestive enzymes. Peristalsis, on the other hand, is the progressive contraction of successive sections of circular smooth muscle resulting in the propulsion of chyme toward the colon. Furthermore, during the fed state the small intestine especially the duodenum exerts negative feedback control on gastric emptying via neural and hormonal mechanisms (secretin, cholecystokinin, and gastric inhibitory peptide).
During the fasting phase, the small intestine exhibits a different pattern of motility characterized by bursts of intense electrical and contractile activity separated by periods of lack of activity. This pattern is called migrating myoelectric complex (MMC). In humans MMC occurs every 90–120 min, originates from the stomach, and sweeps through the small bowel till the terminal ileum. The function of MMC is to clear undigested particles and propagate them to the colon [19].
9.3.1.3 Small Intestinal Dysmotility
Motor disorders of the small bowel can lead to symptoms and signs of “functional” as opposed to mechanical small bowel obstruction. Patients frequently complain of abdominal distension, bloating, and abdominal pain, and when small intestinal dysmotility is associated with gastroparesis, nausea and vomiting may be prominent. Small intestinal dysmotility may be acute or chronic. Acute dysmotility is commonly seen following abdominal surgery, severe septicemia, or electrolyte disturbances such as hypokalemia. Chronic dysmotility is termed pseudo-obstruction (Table 9.5).
Table 9.5
Motor disorders of the small intestine
Cause | Mechanism | Outcome |
|---|---|---|
Acute illness | Altered neurotransmission | Adynamic ileus |
Pregnancy | Decreased smooth muscle contraction (progesterone) | Slow transit |
Diabetes mellitus | Autonomic dysfunction | Slow or rapid transit |
Scleroderma | Smooth muscle fibrosis | Weak contractions |
Neuronal loss in gut wall | Slow transit | |
Primary pseudo-obstruction | Neuronal loss, plexus degeneration | Weak contractions |
Abnormal MMC | ||
Slow transit | ||
Myopathies | Myocyte and mitochondrial abnormalities | Weak segmentation and peristalsis |
9.3.1.4 Malabsorption
Malabsorption syndrome is an alteration in the ability of the GI tract, usually the small intestine, to absorb one or more nutrients adequately from diet into the bloodstream. These may include fats, proteins, carbohydrates, vitamins, or others. This may result from acquired or congenital defects.
Common causes of malabsorption syndrome include inflammatory bowel disease, tropical sprue, Whipple’s disease, lactase deficiency, and parasitic diseases. Other causes are past intestinal surgeries, bacterial overgrowth, gluten enteropathy (nontropical sprue), AIDS, radiation to the abdomen, diabetes, lymphoma, or motility disorders. In addition to small bowel disease, malabsorption can occur in those who have had portions of their stomachs removed surgically. The pancreas produces enzymes that help to digest food, so if a condition exists where enzymes are not being produced, it can result in maldigestion or malabsorption. This could include chronic alcoholic pancreatitis, trauma, cystic fibrosis, tumors, or postsurgical states. The diagnosis of malabsorption syndrome and identification of the underlying cause can require extensive laboratory diagnostic testing. Stool collections and cultures are useful as well as certain breath and hormone tests. Scintigraphic imaging and quantitation is also used for some forms.
9.3.1.4.1 Protein-Losing Enteropathy
Protein-losing enteropathy is a condition in which excess protein loss into the gastrointestinal lumen is severe enough to produce hypoproteinemia. It occurs with many of the previously listed conditions causing malabsorption. Furthermore, diseases such as constrictive pericarditis, congestive heart failure, intestinal lymphangiectasia, nephrotic syndrome, and systemic lupus erythematosus may also cause protein loss from the gastrointestinal tract without any observable mucosal lesions in the bowel. The mechanism of the loss of plasma protein into the gastrointestinal tract in these diseases is not fully understood [20].
9.3.1.4.2 Vitamin B12 Malabsorption
Vitamin B12 deficiency due to pure dietary inadequacy of this vitamin is very rare and occurs mainly in strict vegetarians. More often gastrointestinal disorders, atrophic gastritis, pernicious anemia, congenital lack or abnormality of gastric IF, and total or partial gastrectomy cause malabsorption and consequent deficiency of this vitamin. Diseases involving the distal ileum such as Crohn’s disease, intestinal stagnant loop syndrome, and rarely congenital selective ileal malabsorption with proteinuria (Imerslund-Grasbeck syndrome) may also result in malabsorption of vitamin B12.
9.3.2 The Colon
9.3.2.1 Anatomical and Functional Considerations
The colon is a tubular structure that extends from the ileocecal valve to the anal verge (Fig. 9.1). It measures approximately 1–5 m and consists of the cecum, ascending transverse, descending, sigmoid colon, and rectum. Like the small intestine, the colonic wall consists of the mucosal submucosa, muscularis, and serosa. However, the colonic mucosa lacks villi. Also, unlike the small intestine, the external longitudinal muscular layer is gathered into three flat longitudinal ribbons of smooth muscle called teniae coli. The continuous contractions of the teniae coli cause sacculations on the wall termed haustrations.
The primary function of the colon is to absorb water and electrolytes from its contents and to pack feces until defecation. The motility of the colon is geared toward this function. As stated above, throughout the colon, localized segmental contractions take place and result in mixing chyme. In the cecum and ascending colon, retrograde (antipropulsive) contractions also occur. The net effect of these motility patterns is to slow transit and facilitate the absorption of water and electrolytes. Periodically, massive contractions start in the proximal colon to propel fecal material toward the sigmoid colon where it is stored. One to three times a day, mass contractions sweep the stool toward the rectum. Distension of the rectum by feces initiates the defecation reflex which is mediated by the pelvic nerves and integrated at the level of the sacral spinal cord.
9.3.2.2 Relevant Colon Diseases
9.3.2.2.1 Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) refers to two disorders: Crohn’s disease (CD) and ulcerative colitis (UC). Both conditions are characterized by chronic relapsing intestinal inflammation. UC affects the colon only, and the inflammation is limited to the mucosa and submucosa in most cases. CD can affect the entire gastrointestinal tract from mouth to anus. The inflammation in CD is granulomatous and transmural. Besides inflammation of the gut, IBD is associated with a number of systemic manifestations including anterior uveitis, axial and peripheral arthropathy, primary sclerosing cholangitis, erythema nodosum, and pyoderma gangrenosum.
The etiology of IBD is unknown. However, many of the immunological and molecular mechanisms that mediate inflammation have been elucidated in recent years. Genetic predisposition, mucosal immune dysregulation, and environmental agents seem to play a role (Table 9.6). In genetically predisposed individuals, environmental factors can participate in the disease by inducing a broad immunological response characterized by an imbalance between anti-inflammatory mediators.
Table 9.6
Pathogenesis of inflammatory bowel disease
Genetic predisposition | Environmental factors | Immunological dysregulation |
|---|---|---|
Family clustering | Infectious agents | Proinflammatory mediators |
NOD2 gene variants | Dietary antigens | Anti-inflammatory mediators |
Gene loci on chromosomes 2, 3, 12 | Intestinal commensals |
CD consists of segmental involvement by a nonspecific granulomatous inflammatory process involving all layers of the bowel with skip areas. The disease involves the small bowel in addition to the colon, and rectal sparing is typical. Less commonly it involves the mouth, tongue, esophagus, stomach, and duodenum.
9.3.2.2.2 Acute Appendicitis
The appendix is a diverticulum of an average of 10 cm in adults arising from the posteromedial wall of the cecum. This fact accounts for its variable positions (retrocecal, subcecal, retroileal, pre-ileal, or pelvic) and behind much of the diversity in clinical presentations among patients with acute appendicitis [23].
The pathophysiology of appendicitis begins with obstruction of the narrow appendiceal lumen by causes, including fecaliths, lymphoid hyperplasia (related to viral illnesses such as upper respiratory infections, mononucleosis, or gastroenteritis), gastrointestinal parasites, foreign bodies, and Crohn’s disease. Continued secretion of mucus results in elevated intraluminal pressure, leading to tissue ischemia, overgrowth of bacteria, transmural inflammation, appendiceal infarction, and possible perforation. Inflammation may subsequently extend into the parietal peritoneum and adjacent structures causing abdominal abscesses.
Acute appendicitis is the most common reason for emergency abdominal surgery and must be differentiated from other causes of abdominal pain. The overall diagnostic accuracy achieved by medical history, physical examination, and laboratory tests has been approximately 80 % as the presentation may be atypical. In atypical cases, ultrasonography and computed tomography (CT) may help lower the rate of unnecessary surgeries. The accuracy rates for ultrasonography range from 71 to 97 % and the modality is highly operator dependent and difficult in patients with a large body habitus. The accuracy rate of CT scanning is between 93 and 98 %. However, there is controversy regarding the use of contrast media [23].
9.3.2.2.3 Colorectal Cancer
Incidence of colorectal cancer is highest in developed countries such as the United States and Japan and lowest in developing countries in Africa and Asia. It is the third most common type of cancer in both men and women in the United States. Pathologically most (over 95 %) of colorectal cancers are adenocarcinomas. Surgery, chemotherapy, radiation therapy, and immunotherapy are the lines of treatment. Nuclear medicine has an important role in the follow-up to detect recurrence. FDG PET has an important role as it is more sensitive than computed tomography for the detection of metastatic or recurrent disease and may improve clinical management in more than 25 % of cases [24]. It is of particular importance to differentiate post-therapy fibrosis and inflammation from viable tumor in presacral region.
9.3.2.2.4 Gastrointestinal Bleeding
The localization of the specific bleeding site in patients presenting with acute GI bleeding remains a serious clinical problem. Acute gastrointestinal bleeding (GIB) can be divided into bleeding in the upper (proximal to the ligament of Treitz) or lower tract. If acute upper GIB is a possibility, lavage with a nasogastric tube should identify acute or subacute bleeding. Endoscopy will localize 80–97 % cases of acute upper bleeding; of these 75 % will resolve spontaneously or with conservative medical therapy and 10 % will require surgery. Because of the length and tortuosity of the colon and contamination of fecal matter and blood, endoscopy is not that successful in lower GIB cases. Peptic ulcers are the most common cause of upper GIB; other causes include gastritis, esophageal varices, Mallory-Weiss tear, esophagitis with or without hiatal hernia, and carcinoma [25]. The three leading causes of lower GIB are diverticular disease, angiodysplasia, and colorectal cancer. Other causes include inflammatory bowel disease, ischemic colitis, infectious colitis, and anorectal disease. This bleeding usually resolves spontaneously in 80 % of cases and rebleeds in 25 %. Angiodysplasias account for 20 % of significant lower GIB and tend to rebleed.
Meckel’s diverticulum is a vestige of the omphalomesenteric duct that is present in about 2 % of the population with two thirds younger than 2 years. It is an outpouch usually found on the antimesenteric border of the ileum, 50–80 cm proximal to the ileocecal valve. Ectopic gastric mucosa is present in about 30 % of cases. Nearly all diverticula responsible for rectal bleeding contain ectopic gastric mucosa. Bleeding, which is usually massive and painless, may result from ileal mucosal ulceration due to acid secretion.
Patients with lower GIB should be stabilized and supported while diagnostic studies are performed. Scintigraphy has emerged as the imaging modality of first choice for localizing bleeding sites in the lower gastrointestinal tract. It is more sensitive for slow or intermittent bleeding, which is a common occurrence. If the scan is positive, arteriography can be employed to deliver vasopressin or embolic agents selectively into the bleeding artery.
9.4 Salivary Gland
9.4.1 Anatomical and Physiological Considerations
The major salivary glands include the parotid and the submandibular gland. The parotid gland is located behind the mandible and consists of a superficial and a deep part. The main parotid duct (Stensen’s duct) runs anteriorly to pierce the buccinator muscle, opening on a papilla on the buccal mucosa opposite the second upper molar tooth. The submandibular gland is smaller than the parotid and lies in the submaxillary triangle just below the mandible. The main duct (Wharton’s duct) passes forward and medially to open on a papilla lateral to the frenulum at the base of the tongue. The sublingual glands are situated anteriorly in the floor of the mouth above the mylohyoid muscle, and each gland opens into the oral cavity through several small ducts.
Salivary glands secrete saliva which is a clear, viscous, and watery fluid that contains two major types of protein secretions, a serous secretion containing the digestive enzyme ptyalin and a mucous secretion containing the lubricating aid mucin. Saliva also contains large amounts of potassium and bicarbonate ions and to a lesser extent sodium and chloride ions as well as several antimicrobial constituents, including thiocyanate, lysozyme, immunoglobulins, lactoferrin, and transferrin. Accordingly saliva provides many several functions including antimicrobial activity, mechanical cleansing action, control of pH, removal of food debris from the oral cavity, lubrication of the oral cavity, remineralization, and maintaining the integrity of the oral mucosa.
9.4.2 Pathophysiology of Relevant Conditions
Nuclear medicine-relevant conditions affecting salivary glands are numerous. These include inflammatory, neoplastic, and mechanical disorders affecting the parenchyma and duct system. Xerostomia is defined as dry mouth resulting from reduced or absent saliva flow. It is not a disease but is a symptom of various medical conditions. Xerostomia may result from such conditions as mumps, Sjögren’s syndrome, sarcoidosis, radiation-induced atrophy, and drug sensitivity.
Inflammation of salivary glands usually presents as diffuse enlargement of the glands, unilateral or bilateral. Bilateral enlargement is caused by inflammation (mumps, Sjögren’s syndrome), granulomatous disease (sarcoidosis), or diffuse neoplastic involvement (leukemia and lymphoma). The vast majority of salivary neoplasms occur in the parotid gland. Over two thirds represent benign mixed or pleomorphic adenomas. Warthin’s tumor is another benign tumor that can be bilateral. The malignant tumors include mucoepidermoid carcinoma, adenocarcinoma, and squamous cell carcinoma. Plain films are of limited use for evaluating these tumors. Sialography in conjunction with CT is the preferred technique [26, 27]. The CT sialogram demonstrates the location of the tumor within the gland and also defines any involvement of the deep structures of the neck.
The duct system of the parotid and the submandibular glands can be demonstrated by sialography, and the technique is particularly valuable in the diagnosis of diseases which affect the duct system such as calculus, stricture, and sialectasia.
9.5 Ascites
Ascites is the accumulation of excess fluid within the peritoneal cavity. It is most frequently encountered in patients with cirrhosis and other forms of severe liver disease, but a number of other disorders may lead to either transudative or exudative ascites. Serous effusion into the peritoneum occurs in cases of general edema of both the cardiac and renal type and is sometimes abundant; some fluid may accumulate also in severe anemias and wasting disease. The most severe ascites, however, results from portal obstruction, the most common cause being cirrhosis of the liver. Hepatic vein occlusion (Budd-Chiari syndrome) is also accompanied by gross ascites.
The pathogenesis of ascites is complex, and multiple factors have been postulated to be involved. In cirrhosis the major vascular obstruction is postsinusoidal, and the flow of lymph is considerably augmented. The lymphatic vessels, including the thoracic duct, are dilated but nevertheless appear inadequate to deal with the increased volume of lymph. Fluid oozes from the liver surface; this is called the weeping liver.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree