Clinical Presentation
The patient is a 43-year-old female with an 8-day history of severe low back pain and left leg pain. Painful episode began while bending to remove clothes from a dryer. No lower extremity weakness observed. Negative straight leg raising sign. Normal lower extremity deep tendon reflexes.
Imaging Presentation
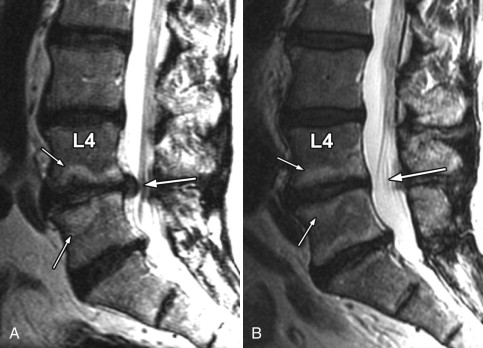
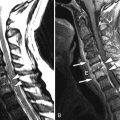
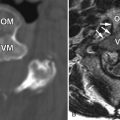
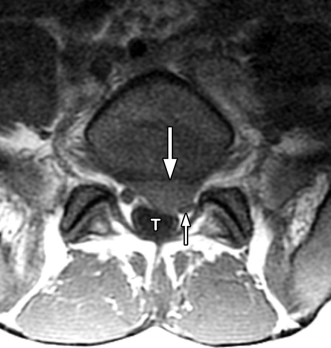
Magnetic resonance (MR) imaging was performed. A large disc herniation (extrusion) was found located on the left at the L5-S1 level. The herniated disc material compresses the left ventral aspect of the thecal sac and displaces the left S1 nerve root posteriorly ( Figs. 22-1 to 22-4 ) .


Discussion
The levels at which 80% to 90% of herniated discs occur are the L4-5 and L5-S1 levels. However, the frequency of disc herniation at a given lumbar level tends to progress in a cephalad direction with advancing age. Disc herniations may extend into the vertebral canal (central canal), lateral recess (subarticular zone), or intervertebral neural canal (neural foramen). Herniated discs may also extend vertically through the vertebral endplates (Schmorl’s nodes). Most herniated discs occur in patients 40 to 60 years of age, but disc herniations may occur rarely in children, sometimes in adolescents, and not uncommonly in young adults and in the elderly.
Possibly, as many as one in three adults are believed to have asymptomatic disc herniations. The majority of patients with acute disc herniation and many patients with chronic disc herniations are symptomatic. The most common manifesting symptoms are low back and radicular pain. Symptomatic patients with radicular pain often have a positive straight leg raising sign (Lasègue sign). If the lumbar herniated disc is large, a cauda equina syndrome may be present. The low back pain associated with lumbar disc herniation is often accentuated by lumbar flexion, sitting, bending, coughing, or sneezing. The back pain often improves if the patient lies flat.
Many factors or conditions may predispose one to the development of disc herniation. Hyperflexion or hyperextension of the spine, lifting heavy objects, and exaggerated twisting movements may all predispose one to disc herniation, particularly if the disc is already internally deranged or disrupted. Acute trauma can also predispose one to disc herniation, although often there is no acute traumatic event that can be related to the onset of symptoms.
Many patients who develop disc herniations also have coexistent internal disc derangement ( disc degeneration ) (see Fig. 22-2; Figs. 22-5 to 22-7 ). The etiology of disc degeneration is controversial, but repetitive microtrauma to the vertebra endplates is believed to interfere with the nutritional supply of the intervertebral disc, which can eventually result in internal disc derangement. Aging factors in the disc including proteoglycan replacement of the nucleus/inner annulus by predominantly type II collagen and metabolic degradation of proteoglycans with concomitant reduction in intradiscal water content ( disc desiccation ) likely play a role as well.



There is a definite familial tendency for development of disc herniation. There is mounting evidence that genetic factors predispose the disc to degeneration (and hence disc herniation). Inherited genetic traits likely render the disc more susceptible to degeneration, disc herniation, or weakening of the vertebral bone. Genes that have been implicated include the following: (1) Those genes associated with the structural integrity of the disc, such as the aggrecan gene, collagen IX gene, or collagen XI gene; (2) those genes that can produce disc matrix-degrading enzymes such as the matrix metalloprotease-3 (MMP-3) gene; and (3) those genes associated with the integrity of bone structure that may be associated with osteoporosis, such as the vitamin D receptor gene and estrogen receptor genes. Recently reported evidence supports a genetic basis for disc degeneration: Polymorphism of the aggrecan gene has been implicated in the development of severe multilevel disc degeneration. Polymorphism of the vitamin D receptor gene may also predispose to disc degeneration. A susceptibility locus for disc herniation and autosomal recessive spastic paraplegia has been found on chromosome 6.
Inflammatory cytokines, such as interleukin-1, may contribute to disc degeneration by causing release of enzymes that degrade proteoglycans. Furthermore, inflammatory cytokines are involved in the mediation of back pain. Enzymes capable of disc degradation, such as matrix metalloproteinases (MMPs) and aggrecanase, are present in normal discs but occur in higher concentrations in degenerating discs and even higher concentrations in herniated discs. These and other enzymes are produced by cells in the disc and also by cells that invade the disc when the disc annulus or vertebral endplates becomes disrupted. It is not certain whether the presence of these substances are causally related to disc degeneration or whether they are produced as a response to disc degeneration or herniation.
Regardless of the underlying cause of disc degeneration, annular fissures (tears) develop in the internally deranged degenerative disc that may facilitate disc herniation, providing a conduit for nuclear and inner annular contents to herniate outside the confines of the disc (see Figs. 22-5 to 22-7 ). Grossly, the herniated disc fragment is usually composed of the disc nucleus, annular tissue, and sometimes portions of the cartilaginous endplate.
A herniated disc often displaces or compresses adjacent structures such as the paraspinal ligaments, thecal sac, cauda equina, and nerve roots within the vertebral, neural foramen, or lateral recess (subarticular zone). Mechanical compression of these structures alone may not be sufficient to generate pain unless the compressed nerves or nociceptors in these tissues adjacent to the disc herniation are sensitized to pain by some other process, which is believed to be some type of inflammation.
When an intravenous contrast agent is administered for MR evaluation of disc herniation, contrast enhancement is nearly always seen in the tissue immediately adjacent to the herniated disc ( Figs. 22-8 to 22-10 ) . The herniated nucleus pulposus in the epidural space causes an inflammatory response in the adjacent tissues manifested by increased vascular permeability and infiltration of the inflamed tissues by leukocytes and macrophages. The precise cause of this peridiscal inflammatory response is not known. When a disc herniates, acidic mucopolysaccarides from the disc nucleus extend through fissures in the annulus into the epidural space, and these metabolites can incite an inflammatory reaction in the epidural space. It is likely that some other biochemical mediator is also released when disc herniation occurs that hypersensitizes the compressed nerve or nearby tissue pain receptors (nociceptors) to the effects of mechanical compression. Many candidates have been proposed for this biochemical mediator of pain including nitric oxide, interleukin-1 (IL-1), interleukin-6 (IL-6), prostaglandin E 2 (PGE 2 ), phospholipase A2 (PLA2), tumor necrosis factor-alpha, granulocyte-macrophage colony stimulating factor, and other substances that have been found in increased amounts within herniated disc material, but their precise role is still unknown. Acidic metabolites released by the herniated disc may also induce an inflammatory response adjacent to the disc fragment.



An autoimmune theory has also been proposed as a cause for the peridiscal inflammation and pain associated with disc herniation. The normal intervertebral disc, lacking a blood supply, is largely sequestered from the immune system throughout most of life. When disc material herniates into the epidural space, which contains richly vascularized immunogenic tissue, the disc material acts like a foreign antigen. If this hypothesis is true, the extruded disc fragment may induce an autoimmune reaction that causes an inflammatory response. Phospholipase A2 may be involved with this process.
Imaging Features
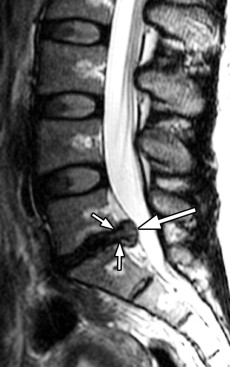
Herniated discs are seen with computed tomography (CT) or MR imaging as focal contour abnormalities along the disc margin. Disc herniations can be subdivided into disc protrusions or extrusions, depending on the size of the base or neck of the disc fragment where it is attached to the outer annulus. A disc protrusion has not penetrated all layers of the annulus (referred to as a contained disc ) and by definition has a relatively wide neck or base relative to the size of the herniated disc apex in the sagittal or axial plane ( Figs. 22-11 to 22-13 ) . A disc extrusion by definition penetrates all layers of the annulus (referred to as a noncontained disc ), and according to currently accepted nomenclature, has a relatively narrow neck (in either the sagittal or axial plane) where it extends through the annulus (see Figs. 22-1, 22-2, and 22-5 ).
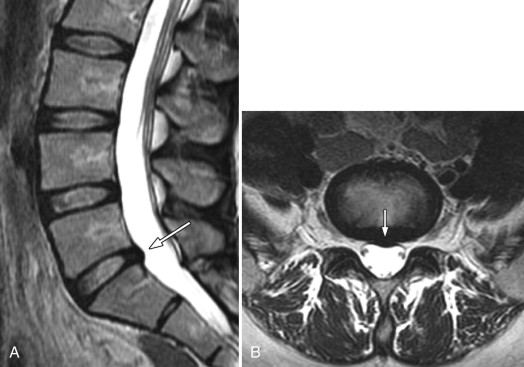
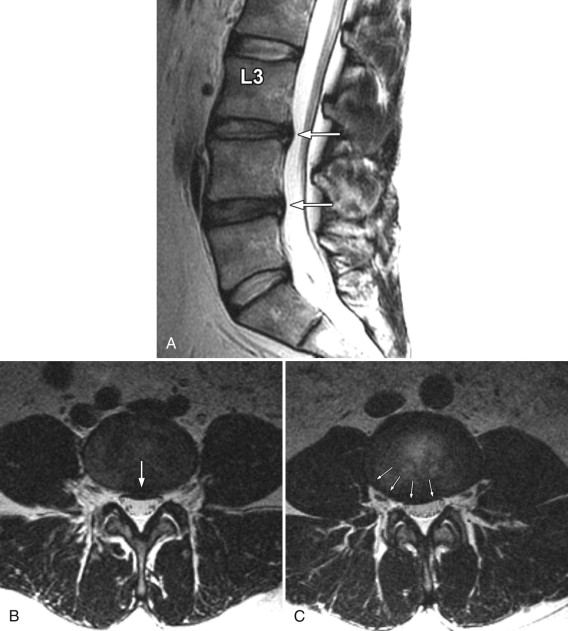

Disc protrusions and extrusions may involute with time, which is usually associated with diminution or resolution of the patient’s symptoms ( Figs. 22-14 ) . Disc extrusions, which are in contact with vascularized tissue in the epidural space, are far more likely to involute than protrusions. Some unknown biochemical process in the epidural space likely causes the disc fragment to involute.