Disease of the Pleura
Barbara L. Knisely
B. L. Knisely: Department of Radiology, University of Wisconsin Hospital and Clinics, Madison, Wisconsin 53792-3252.
THE PLEURA
Pleural diseases are common and encompass a variety of complex appearances depending on histologic features, location, and size. Imaging is integral in the evaluation of pleural disease, aiding in diagnosis and therapeutic interventions. Diseases of the pleura are often first detected and evaluated on chest radiographs. Conventional posteroanterior and lateral views of the chest may be supplemented by lateral decubitus views, and in the past oblique and highly penetrated radiographs were used. However, chest radiography is limited because of its low tissue specificity, and computed tomography (CT) more accurately characterizes pleural disease in terms of tissue composition, location, and extent of disease.99 Ultrasound may direct therapeutic interventions, particularly guided thoracentesis for the detection and localization of pleural fluid, and may allow differentiation between soft-tissue masses and pleural effusions.
Pleural Anatomy
The pleura envelops the lungs with a serous membrane of mesodermal origin, comprising layers of visceral and parietal pleura. A small quantity of fluid lubricates the pleural space, allowing the lungs to readily alter their shape and providing a cushion between the lungs and the chest wall. Parietal pleura lines the ribs, diaphragm, and mediastinum, and visceral pleura covers the lungs and interlobar fissures. The two pleural layers join together at the pulmonary hila and at the inferior pulmonary ligament, becoming continuous. The two lungs contact one another at the anterior and posterior junction lines, representing the interface of the right and left pleural surfaces of the thorax.
The blood supply of the parietal pleura is provided by the systemic circulation, with the visceral pleura being fed by both the pulmonary and bronchial circulations.63,82 Lymphatics supply both the visceral and parietal pleura, but only the parietal lymphatics communicate directly with the pleural space. The pleural lymphatics ultimately drain into the thoracic duct, with a highly variable course of the lymphatic channels along the way. The innervation of the parietal pleura is through intercostal sensory nerves to the costal surface and peripheral diaphragm, with the phrenic nerve supplying the central diaphragm.63 The visceral pleura lacks pain fibers and is relatively insensitive.82,103
Radiographic and Computed Tomographic Features
The pleura cannot be seen on chest radiographs, except where the lungs contact each other at the junction lines and where the visceral pleura infolds to form the fissures. CT and high-resolution computed tomography (HRCT) cannot image the normal pleura, because the pleura cannot be separated from the surrounding structures at the lung–chest wall interface. On HRCT, the intercostal stripe is visualized; it is composed of two layers of pleura, extrapleural fat, endothoracic fascia, and the innermost intercostal muscle.69 The intercostal stripe runs along the lung–chest wall interface overlying an intercostal space, producing a 1- to 2-mm, soft-tissue attenuation, linear opacity connecting the inner margins of adjacent ribs.
PLEURAL EFFUSION
Pleural effusions represent the most common clinical manifestation of pleural pathology.116 A mismatch between the rates of inflow and outflow of fluid in the pleural space leads to a pleural effusion.25 The following mechanisms have been implicated in the formation of pleural effusions, either alone or in combination: (1) increased capillary hydrostatic osmotic pressure, (2) decreased colloid osmotic pressure, (3) increased microvascular permeability, (4) decreased lymphatic pleural drainage, (5) decreased pleural surface pressure, and (6) transdiaphragmatic passage of peritoneal fluid.125
The analysis of pleural fluid allows classification of pleural effusions into transudates and exudates. Transudates result from a decrease in the colloid osmotic pressure, as seen in hypoproteinemia, or from an increase in the microvascular hydrostatic osmotic pressure, with the most important determinant being the systemic venous pressure. The diagnosis of a transudate eliminates the need for further diagnostic workup, because the pleura is normal. Therapeutic management of a transudate depends on the underlying systemic abnormality, which most commonly is congestive heart failure.81 Other common causes for a transudative pleural effusion include cirrhosis, nephrotic syndrome, nephrogenic effusion, hypoalbuminemia, constrictive pericarditis, atelectasis, pulmonary embolism, and myxedema. Given the broad nature of the underlying systemic abnormalities, transudates are often bilateral.
Exudates result from an alteration in the pleural surface, with either an increase in permeability or a decrease in the lymph flow, caused by pleural malignancy or inflammation. The diagnosis of an exudate necessitates further diagnostic procedures to elucidate the underlying cause. An exudative effusion is defined by either a ratio of pleural fluid to serum protein greater than 0.5 or a ratio of pleural fluid to serum lactase dehydrogenase (LDH) greater than 0.6.81,83
Transudates and exudates together comprise the majority of pleural effusions in common clinical settings. Heart failure, cirrhosis, ascites, pleuropulmonary infections, malignancy, and pulmonary embolism account for more than 90% of pleural effusions.70 Bilateral effusions are commonly transudates, but metastatic disease, lymphoma, rheumatoid arthritis, systemic lupus erythematosus, and pulmonary embolism are also causes.115 Very large effusions are commonly seen in metastatic breast and lung carcinoma, heart failure, cirrhosis, tuberculosis, empyema, and hemothorax.87 In a review of symptomatic and asymptomatic pleural effusions, more than 70% of the cases were found to result from heart failure, malignancy, lung infection, or surgery.139
Radiographic Features of Pleural Effusion
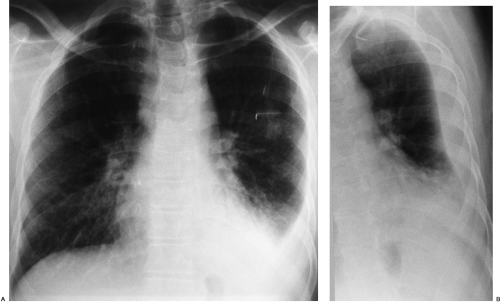
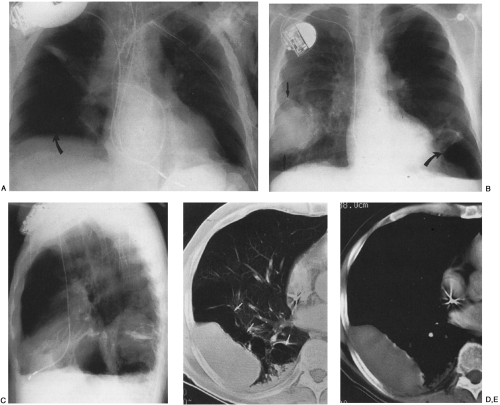
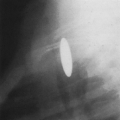
The radiographic appearance of a pleural effusion depends on the patient’s position at the time of the examination and the mobility of the pleural fluid (Fig. 33-1). The most sensitive radiographic projection for identification of pleural fluid is the lateral decubitus chest radiograph, which can detect as little as 5 mL of pleural fluid.98 On the more common posteroanterior chest radiograph, accumulation of at least 200 mL of pleural fluid is needed to cause blunting of the lateral costophrenic angles, and at times 500 mL of pleural fluid may not cause any radiographically apparent blunting.30
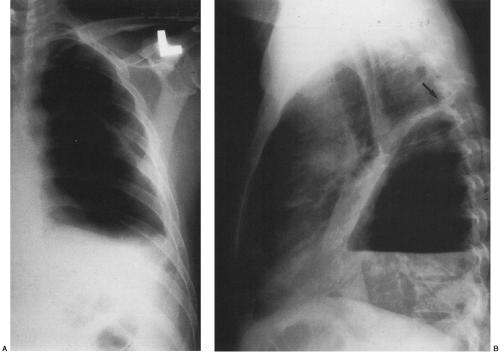
In the erect patient, pleural fluid initially collects in the subpulmonic region.66 Identification of a subpulmonic effusion relies on the presence of the elevated hemidiaphragm sign.156 The superior margin of the fluid collection mimics the contour of the diaphragm. This results in apparent elevation of the ipsilateral hemidiaphragm with flattening of the medial portion of the hemidiaphragm, causing the dome of the diaphragm to appear shifted laterally. Subpulmonic effusions are often transudates, related to hepatic cirrhosis, nephrotic syndrome, renal failure, and congestive heart failure.35,155 Unilateral subpulmonic effusions are more common on the right side.110 When they are bilateral, subpulmonic effusions may be overlooked, given the symmetry of the apparently elevated hemidiaphragms (Fig. 33-2).155 Large pleural effusions may be missed on a supine anteroposterior chest radiograph, because the fluid layers posteriorly. Generally, the volume of fluid in the pleural space is underestimated in the supine patient.123 Capping of the lung apex with pleural fluid is considered an early sign of a pleural effusion in the supine patient, because the apex is the most dependent portion of the thorax tangential to the frontal x-ray beam.114 Other signs produced by pleural effusions in supine patients include increased hazy opacity of a hemithorax with preserved vascular markings, blunting of the costophrenic angle, hazy diaphragm silhouette, thickening of the minor fissure, widened paraspinal soft tissues, and the elevated hemidiaphragm sign.47,67,96,114,121,123,153,164
In the erect patient, pleural fluid initially collects in the subpulmonic region.66 Identification of a subpulmonic effusion relies on the presence of the elevated hemidiaphragm sign.156 The superior margin of the fluid collection mimics the contour of the diaphragm. This results in apparent elevation of the ipsilateral hemidiaphragm with flattening of the medial portion of the hemidiaphragm, causing the dome of the diaphragm to appear shifted laterally. Subpulmonic effusions are often transudates, related to hepatic cirrhosis, nephrotic syndrome, renal failure, and congestive heart failure.35,155 Unilateral subpulmonic effusions are more common on the right side.110 When they are bilateral, subpulmonic effusions may be overlooked, given the symmetry of the apparently elevated hemidiaphragms (Fig. 33-2).155 Large pleural effusions may be missed on a supine anteroposterior chest radiograph, because the fluid layers posteriorly. Generally, the volume of fluid in the pleural space is underestimated in the supine patient.123 Capping of the lung apex with pleural fluid is considered an early sign of a pleural effusion in the supine patient, because the apex is the most dependent portion of the thorax tangential to the frontal x-ray beam.114 Other signs produced by pleural effusions in supine patients include increased hazy opacity of a hemithorax with preserved vascular markings, blunting of the costophrenic angle, hazy diaphragm silhouette, thickening of the minor fissure, widened paraspinal soft tissues, and the elevated hemidiaphragm sign.47,67,96,114,121,123,153,164
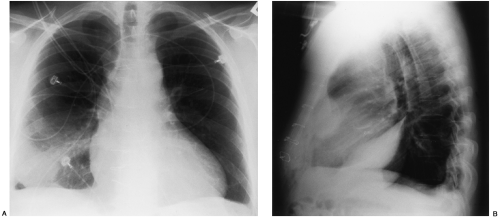
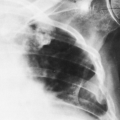
Loculated pleural effusions are accumulations of pleural fluid within the fissures or between the visceral and parietal pleura when the pleural layers are partly adherent (Fig. 33-3).81 Loculated effusions cannot shift freely within the pleural space, and they most commonly form adjacent to the chest wall (Fig. 33-4).81
A loculated pleural effusion may occur within a fissure, particularly in patients with congestive heart failure.44,68,159 Other causes for loculated pleural effusion include the sequelae from exudative effusions, empyema, and hemothorax (Fig. 33-5). A free-flowing pleural effusion may simulate a loculated pleural effusion or a mass if it lies adjacent to the mediastinum or within the interlobar fissures.64 The shift of free fluid that differentiates it from a loculated pleural effusion can be observed by obtaining radiographs with the patient in different positions (Fig. 33-6).64
Computed Tomography of Pleural Effusion
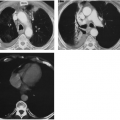
Differentiation of pleural fluid from ascites on CT can be difficult when pleural fluid collects in the posterior costophrenic recess adjacent to the diaphragm. A number of CT signs, including the diaphragm sign,6 the interface sign,150
the displaced crus sign,36 and the bare-area sign,101 have been described to aid in distinguishing between pleural effusion and ascites in the peritoneal cavity. The diaphragm sign defines ascites as being inside the dome of the diaphragm, and pleural fluid outside the dome, in the presence of a noninverted diaphragm.6,101 The diaphragm sign is useful only if the diaphragm can be identified. The location of the diaphragm usually is readily identified when ascites are present but may not be identified when a pleural effusion is present.60 In one fifth of a series of 38 patients with peridiaphragmatic
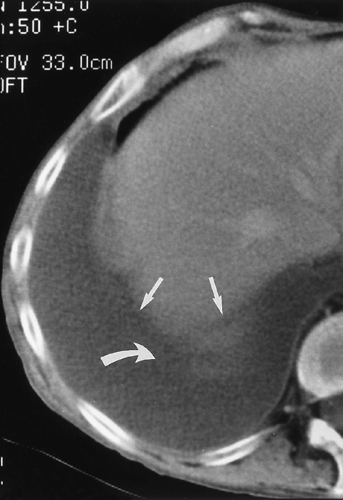
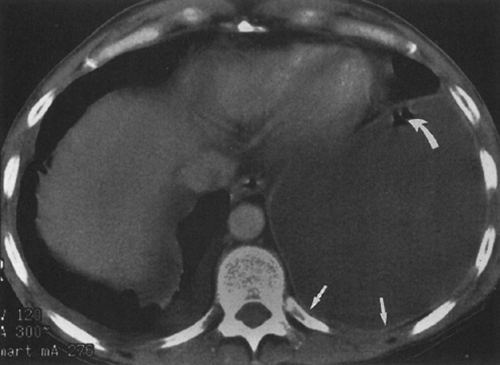
fluid collections, the diaphragm could not be identified.61 A hazy interface between pleural fluid and the liver or the spleen describes the interface sign.150 In the presence of ascites, the interface would be sharp. The displaced crus sign describes anterolateral displacement of the diaphragmatic crus, when pleural fluid collects between the crus and the spine (Fig. 33-7).36,101 A collection of ascitic fluid would result in the opposite displacement of the diaphragmatic crus. In one series, this sign was indeterminate in one third of the cases, limiting its usefulness.61 The bare-area sign uses the restriction of peritoneal fluid by the coronary ligaments along the bare area over the posteromedial surface of the right lobe of the liver, where pleural fluid is free to collect, as a means of differentiating ascites from pleural fluid (Fig. 33-8). The most accurate of these signs in the evaluation of peridiaphragmatic fluid are the interface and bare-area signs.61
the displaced crus sign,36 and the bare-area sign,101 have been described to aid in distinguishing between pleural effusion and ascites in the peritoneal cavity. The diaphragm sign defines ascites as being inside the dome of the diaphragm, and pleural fluid outside the dome, in the presence of a noninverted diaphragm.6,101 The diaphragm sign is useful only if the diaphragm can be identified. The location of the diaphragm usually is readily identified when ascites are present but may not be identified when a pleural effusion is present.60 In one fifth of a series of 38 patients with peridiaphragmatic
fluid collections, the diaphragm could not be identified.61 A hazy interface between pleural fluid and the liver or the spleen describes the interface sign.150 In the presence of ascites, the interface would be sharp. The displaced crus sign describes anterolateral displacement of the diaphragmatic crus, when pleural fluid collects between the crus and the spine (Fig. 33-7).36,101 A collection of ascitic fluid would result in the opposite displacement of the diaphragmatic crus. In one series, this sign was indeterminate in one third of the cases, limiting its usefulness.61 The bare-area sign uses the restriction of peritoneal fluid by the coronary ligaments along the bare area over the posteromedial surface of the right lobe of the liver, where pleural fluid is free to collect, as a means of differentiating ascites from pleural fluid (Fig. 33-8). The most accurate of these signs in the evaluation of peridiaphragmatic fluid are the interface and bare-area signs.61
The sensitivity and accuracy of CT are greater than those of chest radiography for the detection and characterization of pleural effusions. The presence of pleural enhancement on CT imaging alone distinguishes between an exudate and a bland transudate. The split-pleura sign refers to the enhancement of the parietal and visceral pleura on CT when an intravenous contrast agent is administered, consistent with an exudative pleural effusion.145 In contrast, transudates do not usually exhibit pleural enhancement. The presence of parietal pleural thickening represents another differentiating feature; it is more common in the setting of pleural disease causing exudates rather than transudates.10
Ultrasonography of Pleural Effusion
When conventional modalities fail to differentiate between loculated pleural fluid and pleural masses, ultrasound may be useful (Fig. 33-9). Ultrasound is a rapid and relatively inexpensive method for guiding diagnostic interventions, including thoracentesis and biopsy of pleural masses. Real-time sonography is particularly valuable, although it is limited by its operator dependency. The sensitivity of ultrasound in the detection of pleural fluid has not been formally assessed but is probably comparable to that of the lateral decubitus chest radiograph.11
Pleural Effusions Caused by Infection
An empyema represents a type of exudate, characterized by purulent fluid in the pleural space. Analysis of the pleural fluid obtained at thoracentesis is necessary for diagnosis, with one of the three following criteria fulfilled: (1) presence of an organism on Gram stain or culture, (2) grossly purulent fluid, or (3) an elevated leukocyte greater than 5 × 109 cells/L.62,81 The diagnosis of empyema is also made in the presence of a parapneumonic effusion with pH less than 7.0
or glucose less than 40 mg/dL, in which case insertion of a chest tube is indicated.81 Pleural fibrosis may result from inadequate treatment of an empyema. The most common causes for empyema are sequelae of acute bacterial pneumonia or lung abscess, followed by thoracic surgery, trauma, and extrapulmonary spread from osteomyelitis of the spine or subphrenic abscess.62
or glucose less than 40 mg/dL, in which case insertion of a chest tube is indicated.81 Pleural fibrosis may result from inadequate treatment of an empyema. The most common causes for empyema are sequelae of acute bacterial pneumonia or lung abscess, followed by thoracic surgery, trauma, and extrapulmonary spread from osteomyelitis of the spine or subphrenic abscess.62
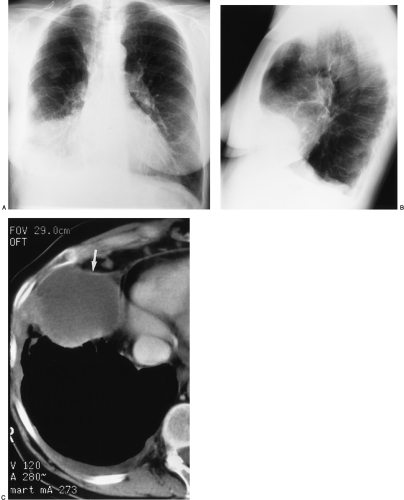
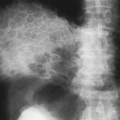
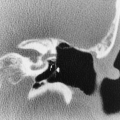
The imaging findings of an empyema on chest radiography include a unilateral pleural effusion or bilateral pleural effusions, with the infected side having the larger volume.62 The lenticular shape and the obtuse angles that loculated empyemas form with the chest wall differentiate them from round abscesses, which have an acute angle with the chest wall (Fig. 33-10).5,62
The CT features of an empyema include the radiographic findings of a lens-shaped fluid collection and obtuse angles with the chest wall, combined with a smooth wall, adjacent lung parenchyma compression, and the split-pleura sign.16,145 Intrapulmonary abscesses, in contrast, have irregular, thick walls that may contain dots of air and that destroy instead of compress the adjacent lung.145 The split-pleura sign, one of the most specific signs for an exudate, describes the enhancement of the thickened visceral and parietal pleura separated by fluid.145 Important CT findings in an empyema include the split-pleura sign, pleural thickening, and air bubbles in the pleural space (Fig. 33-11).145 Air in the pleural space may indicate introduced air from thoracentesis, infection by a gas-forming organism, or a bronchopleural fistula.
The therapeutic interventions for an empyema and for a lung abscess differ, making differentiation of an empyema from a lung abscess important. Chest-tube drainage is indicated in an empyema, whereas lung abscesses require antibiotics and postural drainage. In the past, the primary treatment of empyema was decortication; this is used less frequently today. More aggressive drainage is now employed, with multiple chest tubes, interventional placement of drainage tubes at targeted loculations, and the use of streptokinase/urokinase to break up loculated collections in the pleural space and improve drainage. Decortication may still be warranted in cases of persistent pleural thickening or inadequate drainage on follow-up CT scans.104
Bronchopleural Fistula
A bronchopleural fistula represents a fistulous communication between the pleural space and the bronchial tree. The
most common causes of bronchopleural fistula are necrotizing pulmonary infections and surgical lung resections.62 Other common causes are penetrating and blunt lung injuries, pleural drains, thoracentesis, and ventilator support with end-expiratory positive pressure (PEEP).111
most common causes of bronchopleural fistula are necrotizing pulmonary infections and surgical lung resections.62 Other common causes are penetrating and blunt lung injuries, pleural drains, thoracentesis, and ventilator support with end-expiratory positive pressure (PEEP).111
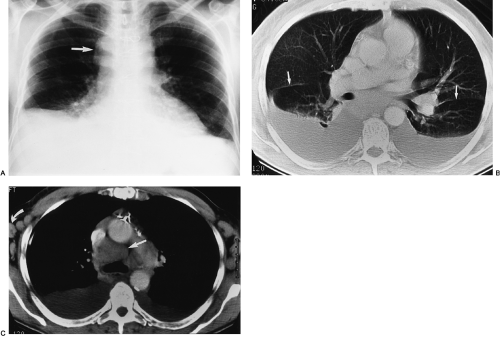
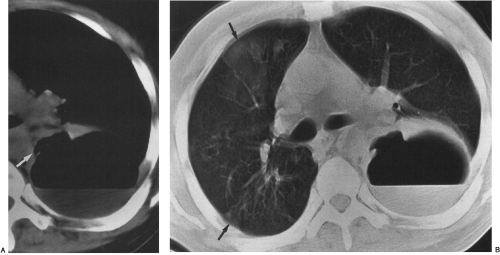
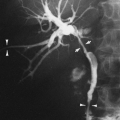
On chest radiography, a bronchopleural fistula may be seen as a hydropneumothorax, an intrapleural air–fluid collection. The pleural location of the air–fluid collection is demonstrated by the extension of the air–fluid level to the chest wall, revealing unequal linear dimensions on orthogonal views. An abscess may be differentiated by an air–fluid level that does not extend to the chest wall and has equal linear dimensions on orthogonal views, consistent with an intraparenchymal location. The imaging modality of choice for demonstration of the communication between the airway and the pleural space in a bronchopleural fistula is CT scanning, including thin-section CT (Fig. 33-12).162 CT scanning provides information regarding the location, size, number, and cause of bronchopleural fistulas that is helpful in treatment planning.147 Current therapeutic interventions for bronchopleural fistulas include pharmacologic therapy for an underlying infection, surgical resection of a destroyed lobe, andsurgical or interventional radiologic occlusion with endobronchial vascular coils or glue.113
Hemothorax
Hemothorax most commonly results from trauma. Less common causes are varicella infection, coagulopathies, and vascular abnormalities.58 In the trauma setting, other causes for a rapidly accumulating pleural effusion must be considered, including iatrogenic causes (e.g., venous line placements), ruptured esophagus, chylothorax, and traumatic subarachnoid-pleural fistula.59 Chest radiographs reveal a pleural effusion without any distinguishing factor to suggest blood in the pleural space. In the acute setting, a noncontrast-enhanced CT scan shows the characteristic increased attenuation (Fig. 33-13) of the pleural fluid.90 Loculations and fibrin bodies may form with the clotting of
pleural blood. Fibrin bodies develop in fibrin-rich fluid, usually after removal or absorption of pleural fluid.27 These fibrin masses are usually solitary, oval or spherical, homogeneous, well-circumscribed, and located in the lung bases.27 Fibrin bodies commonly measure less than 4 cm in diameter, may be fixed or mobile, and may spontaneously disappear or persist unchanged for years.42,151 Early evacuation of the pleural space is indicated to avoid organization of the hemothorax, with extensive pleural thickening (fibrothorax), which typically requires a decortication.
pleural blood. Fibrin bodies develop in fibrin-rich fluid, usually after removal or absorption of pleural fluid.27 These fibrin masses are usually solitary, oval or spherical, homogeneous, well-circumscribed, and located in the lung bases.27 Fibrin bodies commonly measure less than 4 cm in diameter, may be fixed or mobile, and may spontaneously disappear or persist unchanged for years.42,151 Early evacuation of the pleural space is indicated to avoid organization of the hemothorax, with extensive pleural thickening (fibrothorax), which typically requires a decortication.
Chylothorax
Disruption of the thoracic duct may result in a chylous effusion, or chylothorax. Fifty percent of chylothoraces are neoplastic in origin, 25% are traumatic, 10% are miscellaneous, and 15% have idiopathic causes.86,143,154 Lymphoma makes up 75% of the neoplastic lesions. Surgery, in particular cardiac surgery, is the most common form of trauma causing chylothorax. The development of a chylothorax may lead to severe nutritional and immunologic compromise owing to the loss of chyle (lymph of intestinal origin).154 Analysis of the pleural fluid obtained by thoracentesis demonstrates the characteristic finding of elevated triglycerides (110 mg/dL) and chylomicrons (causing a milky appearance).143 Chylothorax usually cannot be differentiated from other effusions based on chest radiographs or CT scans. A single case report of a chylous effusion with low CT attenuation, apparently because of its fat content, has been described.77
Rare causes for chylothorax include lymphatic developmental
anomalies such as lymphangioma, lymphangiectasis, diffuse pulmonary lymphangiomatosis, and lymphangioleiomyomatosis.12 Lymphangioleiomyomatosis, thought to represent a forme fruste



anomalies such as lymphangioma, lymphangiectasis, diffuse pulmonary lymphangiomatosis, and lymphangioleiomyomatosis.12 Lymphangioleiomyomatosis, thought to represent a forme fruste
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree