Pulmonary and Airway Problems in the Pediatric Patient
Mary Ellen Peters
M. E. Peters: Department of Radiology, University of Wisconsin Hospital and Clinics, Madison, Wisconsin 53792-3252.
THE CHEST IN INFANCY AND CHILDHOOD
In the infant, the anteroposterior (AP) diameter of the thorax is relatively greater than in the adult. With growth, the chest decreases in its AP diameter, and the vertical and transverse diameters gradually increase. Also, the diaphragm is higher, making the thoracic cavity relatively smaller. The ribs are almost horizontal in position and gradually angulate downward as the child grows. The sternum is incompletely ossified at birth and ossifies in a segmental manner. The ossification centers are of radiographic importance because they appear as small, rounded opacities that may overlie the lungs in oblique or semioblique projections. They should be recognized as ossification centers and not be mistaken for lesions within the pulmonary parenchyma.
The lungs in the infant and child tend to be slightly more radiolucent than in the adult, because the pulmonary interstitium usually is not visible. The relative size of the visible vascular trunks, however, is comparable. The hilar shadows are relatively high and usually are situated at the level of the third thoracic vertebra. The tracheal bifurcation gradually descends and reaches the adult level (fifth thoracic vertebra) at about 10 years of age. Often the left hemidiaphragm is higher than the right in infants because the stomach is frequently distended with air.
The thymus is a bilobed structure located in the anterior mediastinum that can cause considerable confusion in the interpretation of the chest radiograph of the infant and young child. It is not until the age of 2 to 3 years that the radiograph of the child has an adult-appearing mediastinum, and in some instances persistent visible thymus is seen on the chest radiograph of the older child. The thymus can simulate cardiomegaly, upper-lobe pneumonia, and atelectasis. Additionally, it can appear as a pathologic mass if it is aberrant in location.
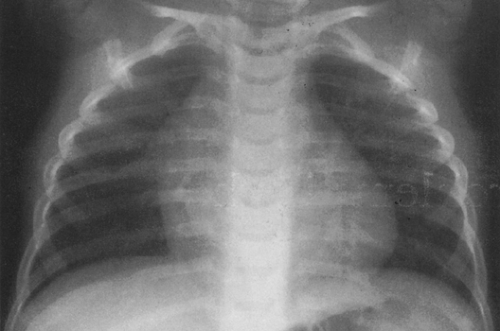
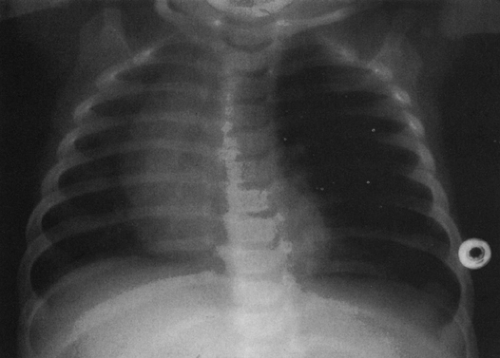
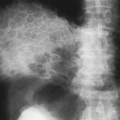
On the frontal view, one lobe of the thymus may extend laterally, producing the “sail sign.” This is more commonly seen on the right (Fig. 23-1). Because the thymus is a soft structure, the ribs can cause indentations on it, as seen on the frontal projection. These undulations are known as the “wave sign” (Fig. 23-2). The thymus may completely obscure the heart on the frontal view, giving the appearance of cardiomegaly. Sometimes a notch can be identified at the most caudal extent of the thymus and a small portion of the cardiac border can be defined (Fig. 23-3A). The lateral view is helpful to determine whether cardiomegaly is present. If the heart is enlarged, it will be seen extending posteriorly (Fig. 23-3B). One other sign that is useful is the “spinnaker sign.” It is seen in the presence of a pneumomediastinum and is caused by air dissecting the lobes of the thymus and displacing them laterally and superiorly off the other mediastinal structures (Fig. 23-4).
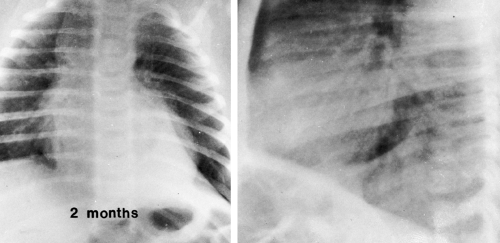
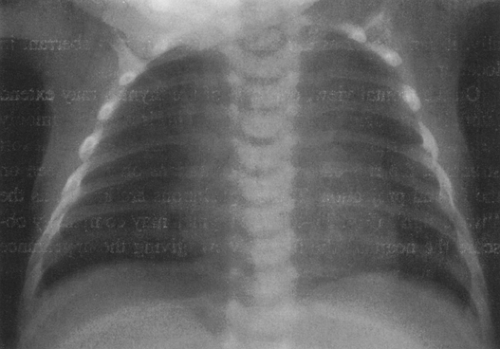 FIG. 23-2.“Wave sign” in a 1-month-old. Note the undulating appearance of the left lobe of the thymus. |
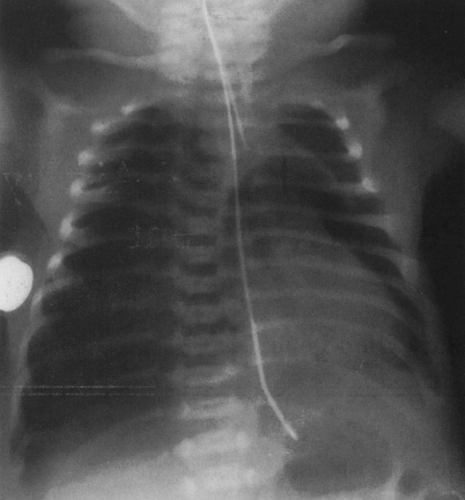 FIG. 23-4.“Spinnaker sign” in a 2-day-old. The thymus (arrow) is displaced off the mediastinum as the result of a pneumomediastinum. A pneumothorax is present on the right. |
Normal thymus may extend posteriorly, simulating a pathologic mass on the chest radiograph (Fig. 23-5A).225 Computed tomography (CT) and magnetic resonance imaging (MRI) can clarify that the mass represents thymic tissue by demonstrating that it is continuous with the thymus in the anterior mediastinum (Fig. 23-5B).206,225 On rare occasions the thymus appears as an isolated ectopic mass in the mediastinum.225 The most common location for ectopic thymus is the neck, where it may be cystic, solid, or a combination of the two.50
With CT and MRI, a normal thymus has uniform homogeneity and enhances uniformly.206 In the infant and child, the thymus has a quadrilateral configuration; the lateral margins are convex outward, straight, or concave on CT and MRI. It is slightly brighter than muscle on T1 and is brighter than fat and muscle on T2.150 At about the time of puberty, the thymus begins to have a triangular shape. A normal thymus
should not have a multilobular appearance.73 In general, a normal thymus does not displace mediastinal structures. However, displacement may be caused by an aberrant thymus.9,198
should not have a multilobular appearance.73 In general, a normal thymus does not displace mediastinal structures. However, displacement may be caused by an aberrant thymus.9,198
Premature infants usually have little or no radiographically identifiable thymus, and this is also true of full-term newborns who are subjected to intrauterine stress. The thymus can involute rapidly during illnesses or systemic stress and rebound in 4 to 8 weeks. Rebound phenomena can also be seen in children after chemotherapy has been discontinued, in hyperthyroidism, and during treatment for hypothyroidism.188,253 A rare occurrence is thymic hyperplasia. It is a benign hypertrophy that occurs in immunologically normal children and is asymptomatic.130,183 In the newborn, the thymus may enlarge secondary to hemorrhage caused by a bleeding diathesis or birth trauma.150 This is commonlyaccompanied by pleural effusion. Other causes for patho-logic thymic enlargement are thymic cyst, hemangioma-lymphangioma, and cellular infiltration in leukemia orlymphoma.67,150
CONGENITAL ANOMALIES
Agenesis and Hypoplasia
Agenesis indicates complete absence of a lobe or lung, including the bronchus and blood supply. In aplasia, there is absence of lung tissue and blood supply, but a rudimentary bronchus is present. These anomalies are very rare; radiographically and clinically the two need not be differentiated.
Agenesis of a lung is usually unilateral and is generally seen on the left.251 It has a high incidence of associated anomalies that are seen more commonly with right-sided agenesis. The anomalies include tracheal stenosis; fused tracheal rings; cardiovascular anomalies, most frequently patent ductus arteriosus and ventricular septal defect; gastrointestinal anomalies, most frequently tracheoesophageal fistula and anal atresia; renal anomalies; vertebral anomalies, most frequently abnormal segmentation of T1-T3; rib anomalies; limb anomalies, most frequently abnormalities of the ipsilateral thumb; and central nervous system anomalies, most frequently anencephaly, microcephaly, and spina bifida.122,168,195 Agenesis of the right lung carries a higher mortality because of a greater mediastinal shift and torsion of the great vessels and bronchi.195 The chest radiograph in agenesis of a lung demonstrates opacification of the involved hemithorax, except for the lucency of the herniated normal lung, which is hyperinflated; ipsilateral shift of the mediastinal structures; and elevation of the ipsilateral diaphragm. The normal lung may appear plethoric because it receives the entire blood supply. If it is not certain whether the radiographic changes represent agenesis, hypoplasia, or atelectasis, CT or MRI may be used to define the anatomy of the bronchial tree and the pulmonary artery. Agenesis of a lobe is most commonly associated with congenital pulmonary venolobar syndrome, which is discussed later.
Pulmonary hypoplasia can be categorized as being either primary or secondary. In both types, bronchial generations and alveoli are decreased to a varying degree, depending on the type of insult and the gestational age at which it occurs. In the secondary type, the hypoplasia may be symmetric, asymmetric, or unilateral. Factors that cause symmetric hypoplasia include oligohydramnios secondary to renal agenesis, obstructive uropathy, or leakage of amniotic fluid; fetal
abdominal masses or ascites; polyhydramnios; high diaphragm secondary to phrenic nerve agenesis and membranous diaphragm; bilateral fetal hydrothorax; and skeletal dysplasias with small thoraces.83,146,195,227 Asymmetric hypoplasia can be the result of an intrathoracic mass or diaphragmatic hernia.104,125,146,227 The ipsilateral lung is more severely affected than the contralateral lung, which is hypoplastic because of a mediastinal shift to that side. Unilateral hypoplasia is seen with stenosis of the tracheobronchial tree and agenesis or hypoplasia of a pulmonary artery.52,146 The radiographic picture in secondary hypoplasia is variable depending on the cause. In unilateral hypoplasia, there is generally very little alteration in the size of the affected hemithorax, because some normal lung tissue remains. Mediastinal shift, elevation of the diaphragm on the involved side, and compensatory overinflation on the opposite side all help to fill the hemithorax.
abdominal masses or ascites; polyhydramnios; high diaphragm secondary to phrenic nerve agenesis and membranous diaphragm; bilateral fetal hydrothorax; and skeletal dysplasias with small thoraces.83,146,195,227 Asymmetric hypoplasia can be the result of an intrathoracic mass or diaphragmatic hernia.104,125,146,227 The ipsilateral lung is more severely affected than the contralateral lung, which is hypoplastic because of a mediastinal shift to that side. Unilateral hypoplasia is seen with stenosis of the tracheobronchial tree and agenesis or hypoplasia of a pulmonary artery.52,146 The radiographic picture in secondary hypoplasia is variable depending on the cause. In unilateral hypoplasia, there is generally very little alteration in the size of the affected hemithorax, because some normal lung tissue remains. Mediastinal shift, elevation of the diaphragm on the involved side, and compensatory overinflation on the opposite side all help to fill the hemithorax.
Primary pulmonary hypoplasia is rare. It has been questioned whether the cause is decreased fetal respiratory activity.226 Presentation is usually within the first 24 hours after birth with hypoxia and tachypnea. The chest radiograph demonstrates small, clear lungs with a high diaphragm. The diagnosis may not be apparent until a series of radiographs demonstrates consistently small lungs.
If the hypoplasia is severe, either in the primary or the secondary form, pneumothorax and pneumomediastinum may be observed.132,227 Usually they occur subsequent to ventilation because the lungs are stiff and difficult to ventilate; however, they may be seen without ventilation. These complications are seen less commonly in cases of skeletal dysplasias and usually do not occur in patients with congenital diaphragmatic hernias and intrathoracic masses until after surgical intervention.227 Persistent fetal circulation is commonly seen in newborns with primary hypoplasia.227 It is the result of hypoxia causing patency of the ductus arteriosus and acidosis causing vasoconstriction and pulmonary hyper-tension. This leads to reversal of shunt through the ductus. Fetal circulation may also be associated with secondary hypoplasia, particularly in those newborns who have congenital diaphragmatic hernia.227
Bronchopulmonary Sequestration
Bronchopulmonary sequestration is a congenital anomaly in which a portion of pulmonary tissue supplied by an arterial branch of the systemic circulation is sequestered from normal bronchial communication, either within a lobe (intralobar sequestration, or ILS) in 75%186 or outside of the normal lung (extralobar sequestration, or ELS). It is one of a spectrum of congenital anomalies termed bronchopulmonary foregut malformations. These anomalies include a wide range of defects: agenesis and hypoplasia of the lung, bronchopulmonary sequestration, aberrant systemic arterial supply to the lung, congenital cystic adenomatoid malformation, bronchogenic cyst, bronchoesophageal and bronchogastric communications, bronchial mucosal rests in the esophageal wall, congenital diverticula of the gastrointestinal tract and bronchi, tracheoesophageal fistula, esophageal atresia, esophageal duplication cyst, and neuroenteric cyst.
Bronchopulmonary sequestration is termed intralobar or extralobar depending on the relationship of the sequestered tissue to the lung. Both types probably have a common embryologic origin, although it has been questioned whether some ILS are acquired.217,235 ILS is an anomaly in which a systemic artery, usually one arising from the lower thoracic or upper abdominal aorta, extends into pulmonary tissue that is not connected to the normal bronchial tree and is therefore termed sequestered. Occasionally the arterial supply is from celiac, splenic, subclavian, innominate, internal thoracic, intercostal, subclavian, inferior phrenic, renal, or pericardiophrenic arteries, or from the ascending aorta.66,172 The arterial supply is multiple in 16% of patients.66 The sequestration usually is drained by the pulmonary venous system, but the drainage may be by the systemic circulation via the intercostal, azygos, or hemiazygos veins, or via the inferior or superior vena cava.66 In 98%, the ILS occurs in a lower lobe, generally on the left.66 There is no gender difference in its occurrence.177 If uninfected, the lesion usually produces no symptoms. In infants there may be sufficient arterial shunting through the sequestration to the pulmonary venous system to cause congestive heart failure, but this is rare.126 The diagnosis is usually made after the age of 20 years when the ILS becomes infected.
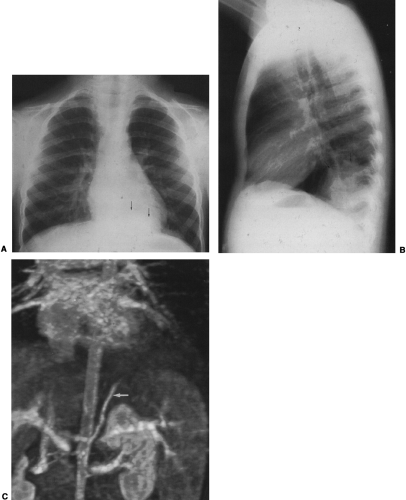
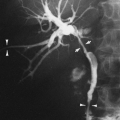
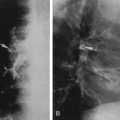
The radiographic findings depend on the presence or absence of infection. In the patients in whom there is no infection, it is usually an incidental finding and is seen as a round or oval mass measuring up to 10 cm or more in diameter. It can also appear as mucoid-impacted, dilated bronchi, or as cystic change. Rarely, it is an area of hyper-radiolucency. It is usually found in the posterior lung base. Occasionally, a poorly defined, finger-like projection extends toward the mediastinum from the medial aspect of the mass, representing the artery supplying the tissue. When infection is present, a fistula to the normal bronchial tree can occur, so that fluid levels are often visible in a single cyst or in several adjacent or multilocular cysts, the walls of which are usually thin. Even though there is evidence of bronchial communication manifested by the presence of air within the cysts, the communication is not ordinarily demonstrated. Normal bronchial branches are usually draped around the mass. Occasionally, calcification may be seen within an ILS.43,111,236 The lesion must be differentiated from an abscess, acquired infected cysts, and chronic pulmonary inflammatory disease with cavitation. The asymptomatic type, with no apparent connection to the bronchi, must be differentiated from tumors and cysts of other origin. The location of these lesions is rather characteristic, however, and when a soft-tissue mass or cyst is noted in the lung base, ILS should be considered (Fig. 23-6A,B). The diagnosis is established when the anomalous artery is demonstrated. Because it is noninvasive and can easily be formatted in multiple planes, MRI is an ideal modality for identifying the artery59 (Fig. 23-6C). Doppler ultrasound
can be useful, particularly in infants, for identifying the vascular supply. CT can demonstrate the cystic portion of the sequestration that may not be identifiable on plain films. High-resolution CT has shown air trapping in the sequestered lung and in the surrounding normal-appearing parenchyma.214 The mucoid-impacted bronchi that may be present within the sequestration can easily be identified with MRI because of high signal intensity on multiecho images.151
can be useful, particularly in infants, for identifying the vascular supply. CT can demonstrate the cystic portion of the sequestration that may not be identifiable on plain films. High-resolution CT has shown air trapping in the sequestered lung and in the surrounding normal-appearing parenchyma.214 The mucoid-impacted bronchi that may be present within the sequestration can easily be identified with MRI because of high signal intensity on multiecho images.151
ELS results when the sequestered tissue is contained in its own pleural covering. It may be located between the lower lobe and the diaphragm, within the diaphragm, in the mediastinum, in the pericardium, in a fissure, attached to the chest wall, or beneath the diaphragm.8,66,120 The arterial supply is similar to that of ILS. It is usually drained by the vena cava or the azygos or hemiazygos venous system, and occasionally by the portal system (in contrast to ILS, which usually drains into the pulmonary venous system). The sequestration is on the left side in 65% to 90% of cases, and it occurs four times more frequently in males.177,186 The diagnosis is commonly made in infancy, because ELS is often associated with other anomalies that include diaphragmatic hernia, eventration, and paralysis; bronchoesophageal communication; congenital cystic adenomatoid malformation; and foregut duplication or diverticula. It also can be associated with polyhydramnios, fetal hydrops, and high-output heart failure secondary to the left-to-right shunt caused by the ELS.66,186 Radiographically, it can appear as a triangular opacity adjacent to the diaphragm, as a paravertebral or a mediastinal mass, or as a bump on the diaphragm (Fig. 23-7). In addition, pericardial effusions and pleural effusions may be seen secondary to an underlying ELS.134,237 The diagnosis is confirmed with demonstration of the arterial supply. CT usually demonstrates a homogeneous mass; however, cystic changes may be present.186 Additionally, pulmonary cysts or emphysema commonly is identified in the lung adjacent to the ELS on CT.111
Congenital Pulmonary Venolobar Syndrome
Congenital pulmonary venolobar syndrome (CPVS) consists of a group of associated anomalies. It is also known as hypogenetic lung syndrome, scimitar syndrome, mirror-image syndrome, epibronchial right pulmonary artery syndrome, and vena cava bronchovascular syndrome.3,74,81 The two most consistent anomalies are partial anomalous venous return and hypogenetic lung.251 Other commonly associated anomalies are bronchopulmonary sequestration, systemic arterialization of the lung, absent or small pulmonary artery, absent inferior vena cava, and accessory diaphragm.3,165,251 Less frequently observed are eventration of the diaphragm, partial absence of the diaphragm, tracheal trifurcation, phrenic cyst, esophageal and gastric lung, horseshoe lung, and anomalous superior vena cava.251 CPVS is almost always right-sided.
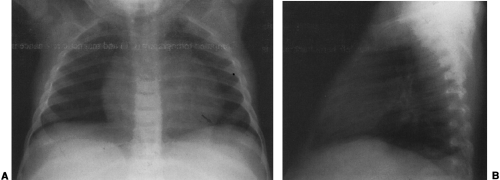
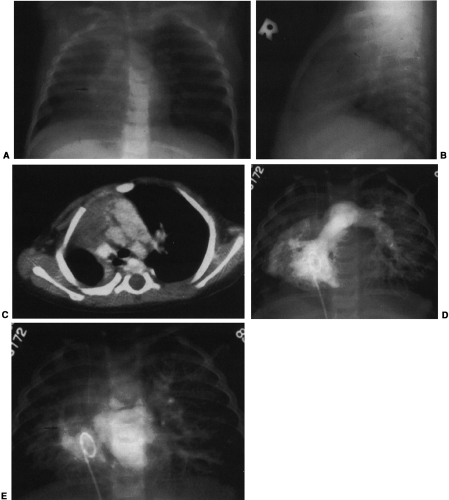
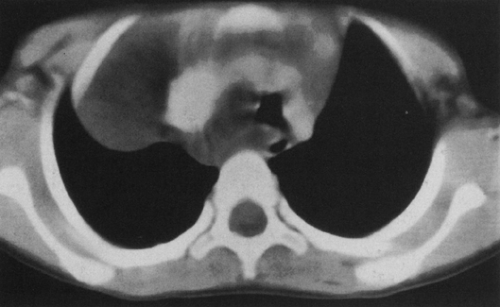
Hypogenetic lung is the result of agenesis, aplasia, or hypoplasia of one or more lobes; the right upper and middle lobes are most commonly involved.251 The frontal radiograph demonstrates the right cardiac border to be ill-defined and the mediastinum to be shifted to the right (Fig. 23-8A). It has been shown by CT that the ill-defined cardiac border is caused by contact of the mediastinum with the lateral chest wall (Fig. 23-8C).3 On the lateral view, a band of increased opacity is seen in the retrosternal area, which is caused by
the mediastinum being outlined by the lung posterior to it (Fig. 23-8B).3 An ipsilateral apical cap and increased opacity at the costophrenic angle may also be seen on the frontal view. These have been shown by CT to be related to subpleural fat.143 If only two lobes are present on the right, the bronchus on the right may mirror the left and be hyparterial.81 Stenosis, diverticula, and atretic bands of the trachea and bronchi may also occur.81 A rare association is tracheal trifurcation, with two main stem bronchi on the right and one on the left.251
the mediastinum being outlined by the lung posterior to it (Fig. 23-8B).3 An ipsilateral apical cap and increased opacity at the costophrenic angle may also be seen on the frontal view. These have been shown by CT to be related to subpleural fat.143 If only two lobes are present on the right, the bronchus on the right may mirror the left and be hyparterial.81 Stenosis, diverticula, and atretic bands of the trachea and bronchi may also occur.81 A rare association is tracheal trifurcation, with two main stem bronchi on the right and one on the left.251
The anomalous partial venous return may involve all or part of the lung. Usually the venous drainage is to the inferior vena cava below the diaphragm. However, it also may enter the portal vein, hepatic vein, azygos vein, supradiaphragmatic inferior vena cava, coronary sinus, right atrium, or, rarely, left atrium.3,251 On the radiograph, the vein may take the form of a curved opacity, which has been likened to a scimitar or a Turkish sword. It may be seen coursing along the right cardiac border, or it may not be identifiable because it is hidden by the heart or is too small (Fig. 23-8E). The anomalous vein can also be straight, or multiple veins may be seen.81
Commonly, the right pulmonary artery is hypoplastic (Fig. 23-8D). However, in a significant number of cases it is of approximately normal size. Rarely is it absent.74 If hypoplasia of the right lung is severe, the vasculature of the left lung is increased.
The associated bronchopulmonary sequestration may be either intralobar or extralobar. Bronchial communication may exist between the sequestration and the esophagus or stomach, forming an esophageal or gastric lung. Even without the presence of pulmonary sequestration, arterialization of all or part of the lung may be present.3,81,102,251 The arterial supply can be from the thoracic or abdominal aorta.
An accessory diaphragm is a thin membrane, occurring in the right thorax, which originates anteriorly with the diaphragm and extends posterosuperiorly to the posterior chest wall.251 All or part of the right middle and lower lobes may be trapped. If the trapped lung is aerated, the membrane may be identifiable. The trapped lung has a high incidence of infection.3,251
Horseshoe lung consists of an isthmus of lung joining the lower lobes behind the heart. The isthmus is usually supplied by the right pulmonary arterial and bronchial systems. Horseshoe lung is associated with malformations of the heart, lungs, and abdominal viscera.61,76 CPVS patients with an associated horseshoe lung have a high incidence of respiratory problems.74,229 On the frontal chest radiograph, the isthmus may be seen as an area of radiolucency at the left medial base. A linear density may be observed, marking its most lateral extent.74 On CT, the isthmus and its associated vessels can be seen extending between the heart and the esophagus.143
Twenty-five percent of patients with CPVS have associated congenital heart disease, with the most common being an atrial septal defect.81,251 Anomalies of the genitourinary tract, skeleton, and eyes as well as omphalocele and mental retardation, may be seen in association with CPVS.251
Bronchogenic Cysts
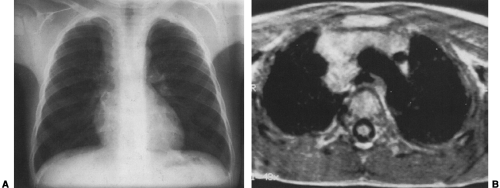
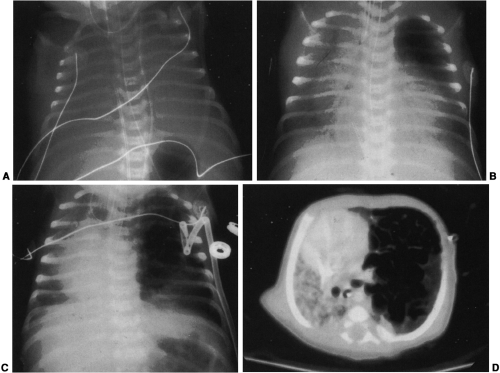
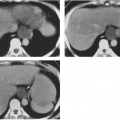
Mediastinal bronchogenic cysts can be a source of respiratory distress in infancy. This is related to the small size of the airways and ease of compressibility of the trachea and bronchi at this age. The two most common locations for mediastinal bronchogenic cysts in infancy are paratracheal and carinal.182 Although a paratracheal bronchogenic cyst can produce sufficient compromise of the tracheal lumen to cause respiratory symptoms, it is the bronchogenic cyst located in the carinal region that is most often symptomatic.60 Unilateral or bilateral hyperinflation, areas of atelectasis, or collapse of an entire lung may be seen on the chest radiograph when a bronchogenic cyst is located in the carinal region (Fig. 23-9A).1,34,91,141,163 Frequently, the cyst itself cannot be detected, only its effects. Presence of the bronchogenic cyst may be demonstrated on an esophagram; it can be confirmed on CT or MRI (Fig. 23-9B).34
Congenital Cystic Adenomatoid Malformation
Congenital cystic adenomatoid malformation (CCAM) is a rare hamartomatous lesion in which there is adenomatoid proliferation of the terminal bronchioles and cyst formation. Usually the involvement is lobar or sublobar; multilobar and bilateral involvement is rare.115,187 The blood supply is predominantly from the pulmonary circulation, but it can be systemic. Three types have been described. Type I, which is the most common, has one or more cysts measuringup to 10 cm in diameter that may be surrounded by smal-ler cysts. In type II, the cysts are more uniform and mea-sure 1 to 2 cm in diameter. Type III, which is the leastcommon, is a large, solid-appearing mass that has micro-scopic cysts, with rare incidence of cysts measuring up to1.5 cm.42,187 All three types have a slightly higher incidencein boys.56,98,187,216
Most patients with CCAM present in the immediate neonatal period with respiratory distress.107,138,187 A small percentage present in later infancy, childhood, or adulthood with recurrent infections or pneumothorax, and in some the CCAM is identified as an incidental finding.78,98,107,171,244 The time of presentation depends largely on the size of the CCAM. Large lesions can cause hypoplasia of the ipsilateral and contralateral lung, resulting in fetal or neonatal death.187 The cysts can enlarge postnatally, causing progressive symptoms.187 Polyhydramnios, placental edema, fetal ascites, and
hydrops are common associated findings, particularly in type III.115,187 Gastrointestinal anomalies and renal agenesis and dysplasia may be seen and are usually associated with type II lesions.115 After surgical resection of the type I CCAM, the prognosis is usually good.56 However, type II lesions have a poor prognosis because of the associated anomalies. The prognosis in type III lesions is also poor because of their large size.56
hydrops are common associated findings, particularly in type III.115,187 Gastrointestinal anomalies and renal agenesis and dysplasia may be seen and are usually associated with type II lesions.115 After surgical resection of the type I CCAM, the prognosis is usually good.56 However, type II lesions have a poor prognosis because of the associated anomalies. The prognosis in type III lesions is also poor because of their large size.56
Radiographically, type I and type II CCAM appear as multiple cysts within a lobe. In some instances, a single large cyst may be the predominant feature in type I.244 Usually the malformation causes mass effect, with a contralateral shift of the mediastinum and atelectasis or hypoplasia of the uninvolved contralateral lung as well as the uninvolved ipsilateral lobe or lobes. The involved lobe may herniate across the mediastinum. In the immediate newborn period, the malformation may appear radiopaque because of retained fetal lung fluid, which eventually drains via bronchial communication.56 Air-fluid levels may be seen within the cysts secondary to fetal fluid or at a later time as the result of an infection. Pneumothorax may occur secondary to rupture of a cyst (Fig. 23-10).78 Type III lesions appear as large solid masses with only a rare occurrence of a macrocyst.
CT is helpful in defining the cystic nature of the mass and the extent of involvement of the lung, and in evaluating the contralateral lung for a second lesion. Ultrasonography is also useful for defining fluid-filled cysts. Additionally, a prenatal diagnosis may be made by ultrasonography, allowing for fetal drainage of the cysts or fetal surgery.35,58,96,98,115
Infantile Lobar Emphysema
Lobar emphysema in infants may not be apparent until weeks or months after birth, rarely, it may be an incidental finding in childhood or in adult life. Therefore, the terms infantile and neonatal are probably more accurate than congenital in referring to this condition. A common cause for lobar emphysema is an abnormality of the bronchus, which causes air trapping. Other causes that have been described are deficiency of cartilage in the bronchial wall, causing it to be flaccid and to collapse on expiration; bronchial stenosis and atresia; bronchial torsion; cartilaginous septa; redundant mucosa; and mucous plugs.18,28,48,75,99,215 Cardiovascular anomalies are commonly associated and may be the source of the lobar emphysema as the result of compression of a bronchus. These anomalies include congenital absence of the pulmonary valve, right or double aortic arch, aneurysm of a pulmonary vein, patent ductus arteriosus, pulmonary artery sling, anomalous pulmonary venous return, and ventricular septal defect.23,192,215 Intrathoracic masses such as bronchogenic cyst, neuroblastoma, teratoma, ELS, and adenopathy can also cause compression of the bronchus leading to lobar emphysema.48,75,99,215 It has been reported in tracheal bronchus and as a sequela of bronchopulmonary dysplasia.46,121 The polyalveolar lobe, in which there are three to five times the normal number of alveoli, is another cause of lobar emphysema.230 In approximately half of the cases, however, no cause is found.48,155
There is a male predominance, with a male-female ratio of 3:1.48 The left upper and right middle lobes are most
often involved. Rarely there is involvement of both lungs, the lower lobes, or more than one lobe. Sublobar emphysema has been described, but it is less common than lobar emphysema.18 In half the cases the clinical presentation is in the first week of life and, of these, one third present at birth. The remainder present before the age of 6 months, with the exception of a very small percentage who present at a later age.122 In some instances, emergency surgery is necessary to remove the overexpanded lobe, but a large number can be managed medically.
often involved. Rarely there is involvement of both lungs, the lower lobes, or more than one lobe. Sublobar emphysema has been described, but it is less common than lobar emphysema.18 In half the cases the clinical presentation is in the first week of life and, of these, one third present at birth. The remainder present before the age of 6 months, with the exception of a very small percentage who present at a later age.122 In some instances, emergency surgery is necessary to remove the overexpanded lobe, but a large number can be managed medically.
Marked hyperlucency is seen on the chest radiograph in the region of the involved lobe. The volume of the lobe is increased and may result in herniation of the affected lobe across the anterior mediastinum, contralateral displacement of the mediastinum, depression of the ipsilateral diaphragm, or separation of ipsilateral ribs. The vascular markings in the affected lobe are widely separated and attenuated, adding to the radiolucency produced by air trapping. Usually the remaining lobe or lobes have some degree of compression atelectasis (Fig. 23-11). In the newborn, the initial presentation may be that of an opaque lobe secondary to retained fetal fluid.65 Linear opacities, which represent dilated lymphatics, may be seen as the fluid clears.2,39,65,75 It has been suggested that many of the cases that present with opaque lobes are of the polyalveolar type.39 The chest radiograph may be all that is needed for the diagnosis. However, CT may be of benefit in demonstrating the cause of the lobar emphysema, and a ventilation-perfusion scan may be helpful to determine the management of the patient by showing the physiologic function of the affected lobe and nonaffected lobes.142,170
Pulmonary Lymphangiectasia
Noonan and coworkers156 classified cases of pulmonary lymphangiectasia into three groups. In the first group are those that are associated with generalized lymphectasia (lymphedema, soft-tissue and bone lymphangiomas, and intestinal lymphangiectasia). Pulmonary involvement in this group is mild, and the prognosis is usually good. The chest radiograph may demonstrate a reticular interstitial pattern and a coexistent pleural effusion. The second group are those that are caused by pulmonary venous obstruction. The two most commonly associated cardiovascular lesions are hypoplastic left heart syndrome and obstructed total anomalous venous return; other causes include cor triatriatum, mitral valve atresia, and pulmonary vein atresia. Because of high pulmonary venous pressure, lymphatic drainage is impaired, causing the lymphatics to become distended. Pulmonary vascular congestion, Kerley B lines, and a reticulonodular pattern as well as pleural effusions and hyperinflation can be seen on the radiograph. The third group is the result of a primary developmental abnormality. The prognosis is poor, and most patients do not survive beyond 24 hours of age. However, a few survive several months or years.193 A reticulonodular pattern is seen on the radiograph, and commonly there is hyperinflation. Kerley B lines, pleural effusions, and pneumothoraces may be present.33,108,135,193,223 Congenital pulmonary lymphangiectasia can have a lobar distribution and can appear as a mass.135
Cystic Hygroma
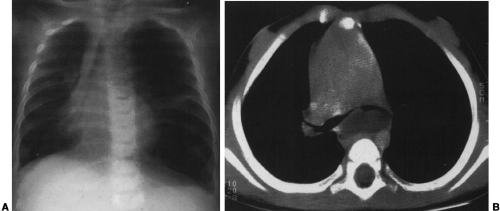
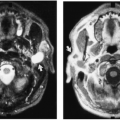
Most cystic hygromas (lymphangiomas) arise in the neck, commonly in the posterior triangle. Of these, 10% extend into the mediastinum.254 Usually they involve the anterior mediastinum; however, they may have peripheral extension of lymphatic channels that spread deep into the mediastinum.202 Rarely, they occur as an isolated mass in the mediastinum. Associated capillary and cavernous lymphangiomas and vascular malformations may be present within the lesion.255 Occasionally, cystic hygromas may be seen in conjunction with generalized lymphangiomatosis. Ninety percent are discovered by the age of 2 years, and 50% are evident at birth.68 Those that extend into the thorax may cause respiratory distress in infancy.
The chest radiograph generally demonstrates a homogeneous mass in the upper thorax with sharp margins and no or little lobulation. An associated soft-tissue mass in the neck usually is easily identified (Fig. 23-12). CT demonstrates a cystic mass which may insinuate between mediastinal structures and extend posteriorly (Fig. 23-13). Enhancement with contrast medium indicates the presence of an associated hemangiomatous malformation. On MRI, most cystic hygromas are heterogeneous, with low signal intensity on T1-weighted images and a higher signal intensity than fat on T2-weighted images.203 Venous aneurysms, which have been described as an associated finding, may be depicted on CT or MRI.116
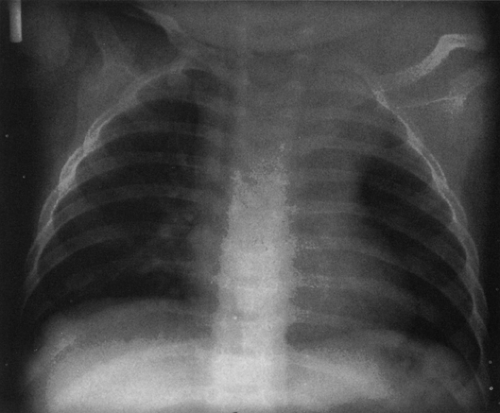 FIG. 23-12.Cystic hygroma. A large, soft-tissue mass is seen in the left neck which extends in to the left upper thorax and displaces the trachea to the right. |
Vascular Ring
Vascular rings that encircle the trachea and esophagus can cause respiratory symptoms including stridor, wheezing, tachypnea, apnea, cyanosis, and recurrent pneumonia. Dysphagia is uncommon. These symptoms may develop in the newborn period.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree