Diseases of Occupational, Chemical, and Physical Origin
John H. Juhl
Janet E. Kuhlman
J. H. Juhl and J. E. Kuhlman: Department of Radiology, University of Wisconsin Medical School, Madison, Wisconsin 53792-3252.
PNEUMOCONIOSIS
The term pneumoconiosis refers to the group of conditions in which solid foreign substances, usually inorganic dusts of varying degrees of pathogenicity, are inhaled and stored in the lung37. These conditions form a group of occupational diseases of considerable economic importance. In some instances there may be enough atmospheric pollution to cause pulmonary disease in people who live near the source. Many foreign materials are capable of producing fibrosis leading to decrease in pulmonary function, and some substances included in this section do not cause significant fibrosis or alteration in pulmonary function. The latter include coal dust (anthracosis), iron oxide (siderosis), barium sulfate (baritosis), and tin (stannosis). Benign changes have also been reported with exposure to titanium oxide and tungsten carbide. Some of these dust diseases occur together, in which case terms such as anthrasilicosis and siderosilicosis are used to designate them. The International Labor Office (ILO) 1980 International Classification System should be consulted when assessing pulmonary disability in patients with industrial or environmental exposure42. This system is also used to classify diseases for statistical or epidemiologic purposes because it provides a quantitative method using standard radiographs for comparison. It is the latest modification of a classification system that has been updated a number of times since 1930.43*
SILICOSIS
Silicosis is caused by inhalation of silicon dioxide particles that are 0.5 to 5 μm or less in diameter. Most particles 5 to 10 μm in diameter are removed from the upper respiratory tract, probably by ciliary action.
According to the US Public Health Service, concentrations of silicon dioxide particles less than 10 μm in size at concentrations lower than 5 × 106 particles per cubic foot do not cause silicosis, whereas concentrations 100 × 106 or higher of similar-diameter particles cause silicosis in all exposed persons. The most active particles in producing the fibrotic reaction are those smaller than 3 μm. When these small particles are deposited in the alveoli, they are ingested by phagocytic cells—the alveolar macrophages. A number of macrophages are killed, stimulating the formation of collagen in the area. Relatively acellular silicotic nodules are then produced in alveoli, respiratory bronchioles, lymphatics, and lymphoid tissue. Some of them remain in the peripheral lymphoid follicles, and others reach the intrapulmonary, bronchial, hilar, and paratracheal nodes. There is some evidence to suggest that an adsorbed protein on the silica particle acts as an antigen, which results eventually in an antibody reaction. This would explain the long latent period as well as the progression of disease long after the patient has been removed from exposure to silica.
Silicosis is found in many industries, including mining, foundries, rock drilling, and grinding involving the production of silica dust. The development of fibrosis requires time. In most occupations, the average time for development of disease in workers exposed to moderate concentrations of silica is 20 years or longer. Accelerated silicosis is said to occur when the exposure has been between 5 and 20 years. When dust counts are unusually high, exposure of less than 5 years can cause acute silicosis. This acute disease, produced by intense exposure over relatively short periods, may
progress to severe respiratory failure and death within 1 year of the onset of symptoms. The patients are often acutely ill, with fever, cough, dyspnea, and weight loss. This type of disease may occur in tunnel workers, sandblasters, and workers involved in mining and milling of silica into a fine powder (silica flour). Roentgen findings are quite different than in simple silicosis. There is a perihilar alveolar process, with air bronchograms. The appearance is similar to alveolar proteinosis, except that it involves the suprahilar areas more than the lung bases. Silicotic nodular opacities are helpful in making the diagnosis, but they may not be present.
progress to severe respiratory failure and death within 1 year of the onset of symptoms. The patients are often acutely ill, with fever, cough, dyspnea, and weight loss. This type of disease may occur in tunnel workers, sandblasters, and workers involved in mining and milling of silica into a fine powder (silica flour). Roentgen findings are quite different than in simple silicosis. There is a perihilar alveolar process, with air bronchograms. The appearance is similar to alveolar proteinosis, except that it involves the suprahilar areas more than the lung bases. Silicotic nodular opacities are helpful in making the diagnosis, but they may not be present.
Radiographic Observations
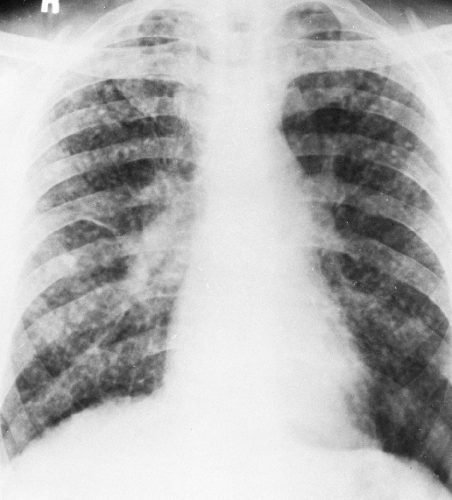
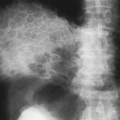
Because the radiographic findings in silicosis early in its development are similar to those in a number of other diseases, it is essential to obtain an adequate history of exposure to make or suggest the diagnosis. Scattered small, round, nodular opacities are found in the upper and central portions of the lung as the initial roentgenographic change (Fig. 27-1). At first the opacities are discrete and small, on the order of 1 to 2 mm in diameter. At this stage, it is likely that an additive effect caused by overlapping nodules is necessary to make them visible. They are usually distributed symmetrically and widely, with a tendency to spare the lung bases. These opacities are usually clearly defined and uniform in density. They may be accompanied by small, irregular opacities, which are fewer in number than the round ones. The nodules gradually increase in size (Fig. 27-2). This may be termed “simple” or uncomplicated silicosis.
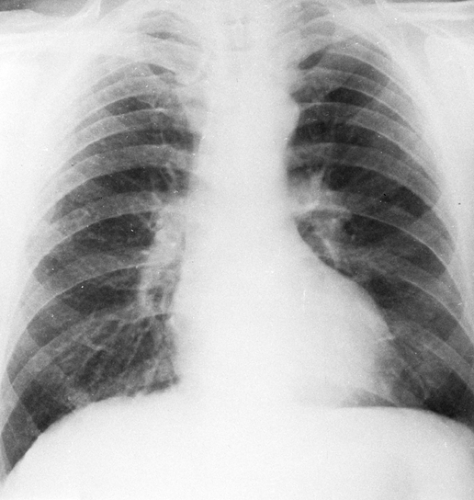 FIG. 27-1. Silicosis. Scattered small nodules are associated with minimal prominence of interstitial markings representing early nodular silicosis. Category I, International Labour Organization classification, 1980.31 |
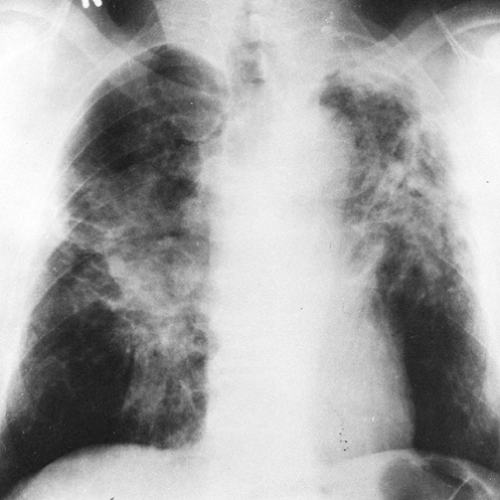
As the pulmonary nodules continue to increase in size, to 1 cm or more in diameter, they may coalesce and tend to become conglomerate (progressive massive fibrosis) (Fig. 27-3). The conglomeration and coalescence are usually accompanied by retraction toward the hilum, leaving the periphery
of the lung overinflated and relatively free of nodules (complicated silicosis). By the time this stage is reached there is often enough emphysema present to cause downward displacement of the diaphragm and a decrease in diaphragmatic motion with respiration. There can be considerable variation in the relative amounts of nodulation, hilar enlargement, and emphysema. Hilar node enlargement is common and may become apparent at any time during the development of the pulmonary abnormalities. The hilar nodes may undergo fibrosis and decrease in size by the time the nodular parenchymal lesions are large enough to be detected readily. Occasionally there is calcification in the silicotic pulmonary nodules. This is a manifestation of long-standing disease. In complicated silicosis, progressive massive fibrosis may ultimately cause migration of the conglomerate nodules toward the hila, and often slightly above them. Large, dense masses are then observed slightly above and lateral to the hilum. Although this process is bilateral, it is not always symmetrical. When it occurs, peripheral emphysema may become very severe. Very few nodules or pulmonary vessels are observed in this hyperlucent peripheral pulmonary tissue.
of the lung overinflated and relatively free of nodules (complicated silicosis). By the time this stage is reached there is often enough emphysema present to cause downward displacement of the diaphragm and a decrease in diaphragmatic motion with respiration. There can be considerable variation in the relative amounts of nodulation, hilar enlargement, and emphysema. Hilar node enlargement is common and may become apparent at any time during the development of the pulmonary abnormalities. The hilar nodes may undergo fibrosis and decrease in size by the time the nodular parenchymal lesions are large enough to be detected readily. Occasionally there is calcification in the silicotic pulmonary nodules. This is a manifestation of long-standing disease. In complicated silicosis, progressive massive fibrosis may ultimately cause migration of the conglomerate nodules toward the hila, and often slightly above them. Large, dense masses are then observed slightly above and lateral to the hilum. Although this process is bilateral, it is not always symmetrical. When it occurs, peripheral emphysema may become very severe. Very few nodules or pulmonary vessels are observed in this hyperlucent peripheral pulmonary tissue.
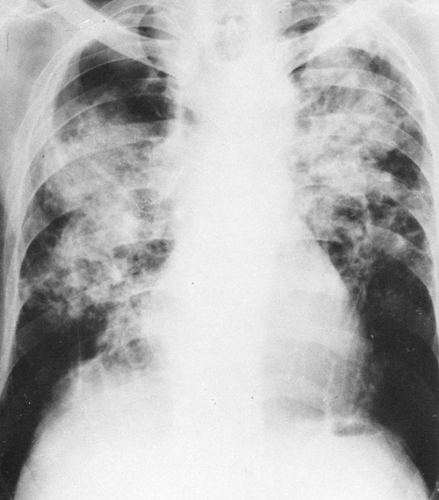 FIG. 27-3. Category C silicosis. There is basal emphysema as well as conglomerate nodular disease (progressive massive fibrosis), which is extensive in both lungs. |
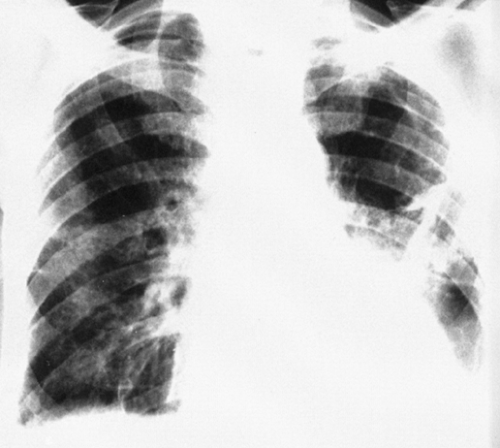
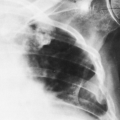
The hilar nodes are sometimes outlined because of the presence of a thin shell of calcium peripherally. This has been termed eggshell calcification, and when present it is very suggestive of silicosis. However, this type of calcification has also been described in patients with no exposure to silica or silicates and in patients with sarcoidosis, postradiation Hodgkin’s disease, blastomycosis, histoplasmosis, scleroderma, and amyloidosis (Fig. 27-4; see Fig. 27-6C). Rarely, an “eggshell” node may erode a bronchial wall and become a broncholith.
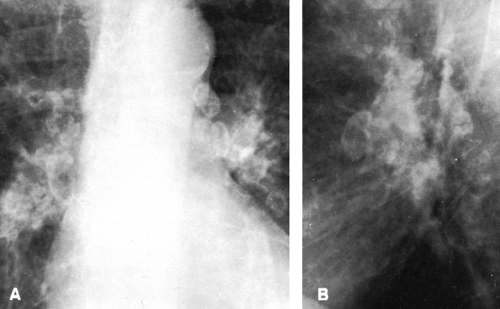 FIG. 27-4. Eggshell node calcification in silicosis is demonstrated in frontal (A) and lateral (B) projections. |
The diagnosis of silicosis can often be suspected on roentgen examination, but the clinical history is of great importance, because the diagnosis cannot accurately be made unless there is a history of enough exposure to silica-containing dust to produce it. Workers in “dusty” industries are often monitored at intervals by means of chest roentgenograms, and review of these serial films often leads to an accurate diagnosis. Extensive roentgen findings can be present without much alteration in pulmonary function, and the reverse is also true in some instances; therefore, there may be lack of correlation between roentgen appearance and pulmonary function.
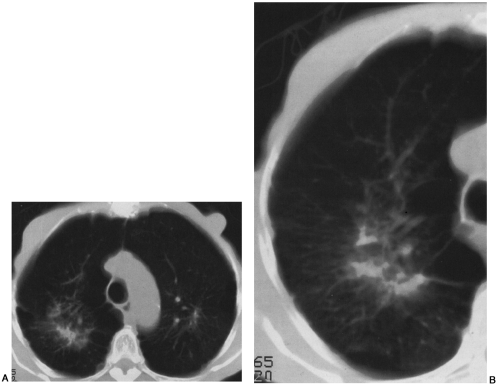
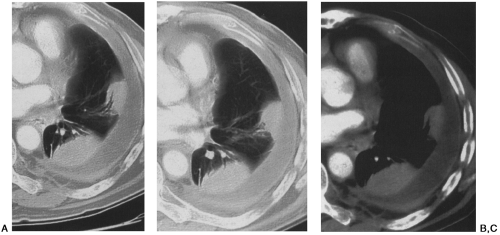
Computed tomography (CT) has been correlated with plain films and pulmonary function tests in silicosis12,81. CT findings of silicosis and coal worker’s pneumoconiosis include micronodules (7 mm or less), macronodules (8 to 20 mm), congolmerate masses, and mediastinal adenopathy often with calcifications (Figs. 27-5 and 27-6)81. High-resolution computed tomography (HRCT) has been shown to be more sensitive than chest radiographs or conventional CT in the detection of micronodules in pneumoconiosis, and we routinely supplement our survey helical CT examination with HRCT slices in patients suspected of having occupational lung disease72,81. Parenchymal nodules in silicosis and coal worker’s pneumoconiosis on CT demonstrate an upper-lobe and posterior predilection12,81. In addition, a typical CT feature of silicosis and coal worker’s pneumoconiosis is the presence of subpleural nodules72,81. Subpleural nodules are seen in only a few other diseases (e.g., sarcoidosis). As disease extent becomes more severe, coalescence of nodules and conglomerate masses are appreciated on CT, as is the surrounding emphysema, fibrosis, and pleural tagging associated with these lesions. CT features of conglomerate masses include irregular margins, calcifications within the masses, bilaterality, and surrounding emphysema72,73,74,81. CT
can be particularly helpful in detecting cavitation within conglomerate masses, which should raise the suspicion for complicating tuberculosis72,81 (see Fig. 27-6).
can be particularly helpful in detecting cavitation within conglomerate masses, which should raise the suspicion for complicating tuberculosis72,81 (see Fig. 27-6).
SILICOTUBERCULOSIS
Tuberculosis is known to complicate silicosis. However, silicosis patients’ immune systems are compromised, so they tend to be susceptible to other infectious diseases as well. Massive areas of density representing conglomerate fibrosis (progressive massive fibrosis) are seen late in the course of silicosis, and some believe that infection is necessary to produce these large masses. The typical location for progressive massive conglomerate fibrosis with or without tuberculosis is above and lateral to the hilum in the infraclavicular part of the lung. The masses are usually bilateral and are often symmetrical in size and location. Usually the mass does not reach to the periphery of the lung; rather, a zone of emphysematous lung is seen lateral to the area of fibrosis, the emphysema developing as the involved lung shrinks with increasing fibrosis.
Atypical forms of conglomerate fibrosis are not uncommon. A mass of fibrous tissue may be present in one lung and not in the other, and the lesions may occur in areas other than the subclavicular zones. Progressive massive fibrosis is often present with little or none of the characteristic nodulation of silicosis in the remainder of the lung, so when massive fibrosis is observed in the presence of nodular silicosis, pulmonary tuberculosis should be suspected (Fig. 27-7). Cavitation occurs in silicotuberculosis, but it is also observed in the absence of infection. Bacteriologic confirmation is necessary but is sometimes very difficult to obtain. In the absence of positive bacteriologic findings, silicotuberculosis should be suspected when the roentgenograms reveal large conglomerate masses in the upper lung and cavitation is present, when the disease is asymmetrical, and when there is a considerable amount of pleural disease. Patients with such roentgenographic findings should be monitored carefully by means of frequent chest roentgenograms and examinations of sputum and bronchial aspirates to exclude tuberculosis. Cavitation in conglomerate nodular silicosis, as noted previously, is not always indicative of tuberculosis. In one series
of 182 patients with cavitation, 18% were found to be nontuberculous. In these patients the cavity evidently resulted from ischemic necrosis within the conglomerate mass.
of 182 patients with cavitation, 18% were found to be nontuberculous. In these patients the cavity evidently resulted from ischemic necrosis within the conglomerate mass.
COAL WORKER’S PNEUMOCONIOSIS
Coal worker’s pneumoconiosis occurs in coal miners and in those who work with coal elsewhere in extremely dusty conditions, such as in the holds of coal barges or ships. The condition is found chiefly in anthracite (hard coal) workers. The disabling pneumoconiosis is usually caused by silica and is, in reality, anthrasilicosis. There is a considerable variation in the amount of quartz (silica) in coal, so the disease varies somewhat in groups of miners who work in different mines. When most of the exposure is to coal dust with little silica, there is little tendency toward progressive massive fibrosis, and large nodules appear to represent the increasing size of small nodules rather than the coalescence of small nodules. There is less diminution of pulmonary function and fewer symptoms. Roentgen findings consist of granular-appearing nodules in the uncomplicated benign form; they are less dense than silicotic nodules. Progressive disease leading to massive fibrosis with nodules or masses that arise peripherally in the upper lungs and tend to migrate toward the hila is found in about one third of those with coal worker’s pneumoconiosis. The characteristic appearance consists of (1) a flat lateral border which is often elongated and parallels the rib cage; (2) a thin mass in the sagittal plane; (3) thick-walled, eggshell calcifications within the mass; and (4) multiple satellite nodules97. Cavitation may develop as a result of necrosis or tuberculosis; however, the cause is not definitely known. In some instances there may be enough silica to be a factor; in others, the condition may be caused or accentuated by infection such as tuberculosis.
Caplan’s Syndrome
Caplan’s syndrome is the combination of coal worker’s pneumoconiosis with rheumatoid arthritis63. Roentgen findings consist of rounded, peripheral nodules from 0.5 to 5.0 cm in diameter, which are clearly defined and may cavitate, on a background of nodular pneumoconiosis. This syndrome usually occurs in patients who have subcutaneous rheumatoid nodules but do not necessarily have arthritis. The pulmonary nodules may appear at intervals and often portend exacerbation of arthritis. They are similar to the necrobiotic nodules found in rheumatoid lung in patients without pneumoconiosis. This syndrome is uncommon, if not rare, in the United States but is evidently more common among coal miners in Wales.
ASBESTOSIS
Asbestos, a hydrated magnesium silicate, is a fibrous mineral used as an insulator against heat and cold and as a fireproofing material. The most important form from the standpoint of pneumoconiosis is the serpentine (wavy) mineral, chrysotile (white asbestos), which is magnesium silicate. This makes up 90% of the asbestos used in the United States and Canada. The other important forms (straight or amphibole) are amosite (brown asbestos), an iron magnesium silicate;
anthophyllite, produced largely in Finland; and crocidolite (blue asbestos), an iron sodium silicate with very fine fibers that appears to have more carcinogenic properties than chrysotile, particularly as a cause of mesothelioma. In many industries, combinations of asbestos fibers are used, which creates problems in the study of relative carcinogenicity. Occupational exposure occurs in the mining industry and in the manufacture and installation of insulating materials containing asbestos. Asbestos exposure also occurs in ship building, in the automotive industry (gaskets, brake linings, undercoating), and in the manufacture of certain “paper” products (roofing felt, flooring felt) and textiles.
anthophyllite, produced largely in Finland; and crocidolite (blue asbestos), an iron sodium silicate with very fine fibers that appears to have more carcinogenic properties than chrysotile, particularly as a cause of mesothelioma. In many industries, combinations of asbestos fibers are used, which creates problems in the study of relative carcinogenicity. Occupational exposure occurs in the mining industry and in the manufacture and installation of insulating materials containing asbestos. Asbestos exposure also occurs in ship building, in the automotive industry (gaskets, brake linings, undercoating), and in the manufacture of certain “paper” products (roofing felt, flooring felt) and textiles.
A number of conditions result from exposure to asbestos, including (1) asbestos-related pleural effusions, (2) asbestos-related pleural thickening and pleural plaques, (3) rounded atelectasis, (4) mesothelioma, (5) lung cancer, (6) gastrointestinal cancer, and (7) pulmonary asbestosis (Figs. 27-8, 27-9, 27-10, and 27-11). The malignant pleural manifestations of asbestos exposure are more fully discussed in Chapter 33.
Pulmonary asbestosis is defined as chronic interstitial pneumonia with diffuse interstitial fibrosis caused by inhaled asbestos fibers. The term pulmonary asbestosis excludes pleural disease such as pleural plaques, diffuse pleural thickening, and pleural effusion—which may, however, indicate prior asbestos exposure.
Although asbestos fibers can be detected in the lungs of many urban dwellers, in Houston the amount was found to be very low and was below the limits of detectability in some residents26. In contrast, small numbers of ferruginous bodies (asbestos bodies) were found in residents of San Francisco20. Usually there is no tissue reaction and only a minor amount of fibrosis, so this level of exposure probably poses no risk. However, among people exposed in the neighborhood of asbestos mines and mills and those who repeatedly handle the clothing of asbestos workers, there is some risk of developing asbestosis and asbestos pleural disease. The extent of the risk has not been completely established.
Pathogenesis
The mechanical irritation of the long, stiff fibers when they become lodged in the lungs is believed to account, at least in part, for the fibrosis that results. There is circumstantial evidence to support a proposed autoimmune hypothesis
for the pathogenesis in this disease, as in silicosis. The silicates are more soluble than silica, so the fibrosis may be a response to the silicic acid and metallic ions released in solution. Bronchoalveolar lavage in pulmonary asbestosis reveals that first there is an alveolitis with an increase in alveolar macrophages, lymphocytes, and neutrophils. Fibroblast replication is accelerated, eventually leading to fibrosis, which is typical of asbestosis. The changes occur in the respiratory bronchioles and alveolar ducts, and the fibrosis is peribronchiolar in location. The cause of the pleural changes is not entirely clear. However, there is some evidence to show that the pleural reaction is at least in part caused by mechanical irritation by the fibers that penetrate the visceral pleura.
for the pathogenesis in this disease, as in silicosis. The silicates are more soluble than silica, so the fibrosis may be a response to the silicic acid and metallic ions released in solution. Bronchoalveolar lavage in pulmonary asbestosis reveals that first there is an alveolitis with an increase in alveolar macrophages, lymphocytes, and neutrophils. Fibroblast replication is accelerated, eventually leading to fibrosis, which is typical of asbestosis. The changes occur in the respiratory bronchioles and alveolar ducts, and the fibrosis is peribronchiolar in location. The cause of the pleural changes is not entirely clear. However, there is some evidence to show that the pleural reaction is at least in part caused by mechanical irritation by the fibers that penetrate the visceral pleura.
The disease does not develop unless there is a lengthy exposure, usually 10 years or more, to a fairly high concentration
of dust. When the pulmonary lesion is established, it progresses even though exposure is not continued. The clinical findings are those of progressive dyspnea which is often out of proportion to the amount of change noted on the chest films. There is often cyanosis and cough with sputum in which asbestos bodies can be detected. However, many patients with asbestos fibers in the lungs are asymptomatic. The incidence of tuberculosis is not as high as in silicosis. There is an increased incidence of carcinoma of the lung in patients with asbestosis. In patients with asbestos exposure who smoke, lung cancer is 70 times more likely than in nonsmokers who have no asbestos exposure. The lung tumors often develop in areas of fibrosis and are therefore extremely difficult to detect at an early stage. Insulation workers in building trades are six to seven times more likely to develop cancer of the lung or pleura than those not exposed to asbestos. This high incidence is found despite the relatively light and intermittent asbestos exposure in this group. Increased incidences of gastrointestinal cancer and of pleural and peritoneal mesothelioma have also been established. However, in one report of 36 cases of mesothelioma, 19 were not associated with asbestosis38. This is an unusual figure, however, because other reports indicate that only 11% to 16% of patients with malignant mesothelioma do not have a history of exposure to asbestos.
of dust. When the pulmonary lesion is established, it progresses even though exposure is not continued. The clinical findings are those of progressive dyspnea which is often out of proportion to the amount of change noted on the chest films. There is often cyanosis and cough with sputum in which asbestos bodies can be detected. However, many patients with asbestos fibers in the lungs are asymptomatic. The incidence of tuberculosis is not as high as in silicosis. There is an increased incidence of carcinoma of the lung in patients with asbestosis. In patients with asbestos exposure who smoke, lung cancer is 70 times more likely than in nonsmokers who have no asbestos exposure. The lung tumors often develop in areas of fibrosis and are therefore extremely difficult to detect at an early stage. Insulation workers in building trades are six to seven times more likely to develop cancer of the lung or pleura than those not exposed to asbestos. This high incidence is found despite the relatively light and intermittent asbestos exposure in this group. Increased incidences of gastrointestinal cancer and of pleural and peritoneal mesothelioma have also been established. However, in one report of 36 cases of mesothelioma, 19 were not associated with asbestosis38. This is an unusual figure, however, because other reports indicate that only 11% to 16% of patients with malignant mesothelioma do not have a history of exposure to asbestos.
Radiographic Observations
The fibrosis produced by the foreign material results in the appearance of small, irregular opacities, primarily in the lung bases as the earliest finding (see Figs. 27-8 and 27-9). As the number of these opacities increases, there may be an accentuation of interstitial markings extending into the perihilar regions and bases. Later there is an increase in the basal fibrotic changes, which usually appear stringy, irregular, and reticular. The cardiac borders assume a shaggy, poorly defined appearance as a result of a combination of interstitial fibrosis and small, irregular opacities. Increasing fibrosis may lead to large opacities, usually when interstitial fibrosis is extensive. This is not very common in asbestosis, however. The lung bases are usually involved, and in some instances the disease may involve the central and upper lungs as well. As a result of extensive basal disease, the outline of the diaphragm may become poorly defined. Cavitation and peripheral emphysema with central conglomeration (as seen in silicosis) does not occur.
HRCT is capable of detecting pulmonary parenchymal abnormalities in 96% of patients with clinical asbestosis1,2. Most CT findings, however, are not specific for asbestosis and can be seen in a variety of other lung diseases and in patients without exposure to asbestos11. CT findings that have been identified in patients with clinical asbestosis include dense fibrotic parenchymal bands, thickened interlobular septa and intralobular lines, thickened centrilobular core structures, architectural distortion of secondary pulmonary lobules, pleural-based nodular irregularities and subpleural dots, subpleural curvilinear lines, peripheral interstitial lines or reticulation, and honeycombing5,6,32,55,56,58,65 (see Fig. 27-9). Although changes of interstitial thickening and honeycombing on CT are not specific for pulmonary asbestosis and can be seen with any cause of interstitial fibrosis, their identification usually in conjunction with asbestos-related pleural plaques is strongly suggestive of asbestosis in a patient with a significant exposure history and an appropriate latency period before the onset of symptoms. The roles of CT and HRCT in the diagnosis of asbestosis remain controversial, however. There is little debate that CT is more sensitive for detection of pleural plaques and parenchymal changes of interstitial fibrosis than chest radiographs in patients exposed to asbestos,32,58 but the significance particularly of minimal or mild parenchymal abnormalities detected by HRCT is hotly contested. Standardization of HRCT criteria for diagnosis of asbestosis has not been established, and a lack of consensus among radiologists and pulmonologists as to the ultimate role of CT in establishing a diagnosis of asbestosis remains. Definitive diagnosis of asbestosis is still a pathologic determination requiring histologic evidence of peribronchiolar fibrosis in association with asbestos bodies in the lung.58
Other chest radiograph and CT findings seen in patients with a history of asbestos exposure include pleural thickening, pleural plaques, and rounded atelectasis (see Figs. 27-10 and 27-11). The pleural changes related to asbestos exposure occur independently and are often observed when no parenchymal disease can be detected. The following pleural manifestations may occur alone or in combination with the others: diffuse pleural thickening, pleural plaque formation, calcification in pleural plaques, and pleural effusion. Diffuse pleural thickening, which may extend into the fissures, is a common roentgenographic finding; it is usually bilateral and is more likely to be associated with interstitial fibrosis than the combination of pleural plaques and interstitial fibrosis. Diffuse pleural thickening involves the visceral pleura and can be recognized as such only when it extends into the interlobar fissures, where there is no parietal pleura. It may encase the entire lung. In asymptomatic patients, the presence of bilateral pleural thickening has a high predictive value for previous asbestos exposure—about 80% when patients with known causes (other than asbestos) for pleural disease are excluded.
Parietal pleural plaques are often the only findings in asbestos-related pleural disease. The plaques vary in thickness from 1 to 10 mm. Because they often occur posterolaterally or anterolaterally in the midthorax, oblique films may be very useful in demonstrating them. However, oblique films also may be confusing and are more useful in demonstrating involvement of the major fissures in patients with diffuse pleural thickening. In problem cases CT is very useful, because it not only demonstrates the pleural plaques but also differentiates them from fat, which may cause confusion on plain films. Diaphragmatic plaques in the absence of calcification are more difficult to identify on CT, because the plane
of sectioning is no longer perpendicular to the plaque. Plaques appear early as thin, local areas of pleural thickening in the midthorax, and they are often overlooked until they enlarge and become thicker. Fissural (visceral pleura) pleural thickening is common in asbestos exposure, and its presence may indicate pulmonary asbestosis even when the chest x-ray is normal77. As indicated previously, asbestos-related pleural thickening is more easily detected on CT than on chest radiography, and extrapleural fat can readily be differentiated from thick pleura. HRCT has been shown to be more accurate than CT in detecting pleural changes. It can identify reduced lung function, indicative of restrictive lung disease, in patients with asbestos exposure and normal chest radiographs.87
of sectioning is no longer perpendicular to the plaque. Plaques appear early as thin, local areas of pleural thickening in the midthorax, and they are often overlooked until they enlarge and become thicker. Fissural (visceral pleura) pleural thickening is common in asbestos exposure, and its presence may indicate pulmonary asbestosis even when the chest x-ray is normal77. As indicated previously, asbestos-related pleural thickening is more easily detected on CT than on chest radiography, and extrapleural fat can readily be differentiated from thick pleura. HRCT has been shown to be more accurate than CT in detecting pleural changes. It can identify reduced lung function, indicative of restrictive lung disease, in patients with asbestos exposure and normal chest radiographs.87
Calcification also occurs in pleural plaques. It is noted most frequently over the diaphragm in the form of a thin, curvilinear opacity conforming to the upper surface of the diaphragm bilaterally and is virtually pathognomonic. Oblique and lateral projections are useful in its detection. Unilateral pleural calcification, particularly when it extends upward along the chest wall, usually indicates previous infection, not asbestos exposure.
Bilateral or unilateral pleural effusion can also occur, but the possibility of other causes must be excluded before effusion can be attributed to asbestosis. The effusion tends to be relatively small in amount (i.e., less than 500 mL), and it may be bloody or blood-tinged. It is often the earliest sign of asbestos-related disease and may be accompanied by pleural pain. In some instances, the effusion contributes to or causes diffuse pleural thickening. Multiple recurrences appear to be rare, but a single recurrence of fluid is observed in almost one third of patients.60
The relative incidence of pleural findings has been studied by McLoud and coworkers61. In a group of 1,373 exposed persons, 16.5% had pleural plaques and 13.5% had diffuse pleural thickening. In those with diffuse thickening, the radiographic findings appeared to indicate that it was caused by the residuals of effusion in 31.4%, represented confluent plaques in 25.4%, and was accompanied by pulmonary fibrosis in 10.3%. Malignancy and infection accounted for 25% of pleural thickening, and in 5% it was caused by obesity. As in silicosis, the diagnosis is based on correlation of the roentgen findings with the clinical findings, plus an accurate occupational history of exposure to asbestos61 (see Figs. 27-8 and 27-10).
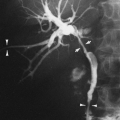
Rounded atelectasis is another abnormality often found in patients with diffuse pleural thickening due to asbestos exposure, although not all cases involve asbestos-related pleural disease1,2,95. Rounded atelectasis can often be suspected on the chest radiograph by the presence of a rounded peripheral opacity, typically in the posterior lower aspect of the lungs in association with pleural thickening. Serial chest films over time may show that the opacity is preceded by a pleural effusion and develops as the effusion resolves to pleural fibrosis. CT is very helpful in establishing the diagnosis of rounded atelectasis. The CT findings include a rounded or lentiform opacity that is in contiguity with an area of diffuse pleural thickening (see Fig. 27-11). Associated volume loss in the adjacent lung and the characteristic “comet tail” of vessels and bronchi leading into the opacity are also characteristic1,2. The need for biopsy may be obviated if these CT criteria are met in the diagnosis of round atelectasis versus tumor.1,2,95
TALCOSIS
Talc is a hydrous magnesium silicate in which there is no free silica. If no asbestos or silica is present, talc causes very little pulmonary dysfunction and minor radiographic changes. Widely scattered, small, poorly defined nodules have been reported in patients who inhaled cosmetic talcum powder, which contains no impurities. Other reports of changes in miners or workers in milling operations involving pure talc indicate minimal basal radiographic findings consisting of small, irregular nodules.
When asbestos is present in talc, it causes pneumoconiosis in workers exposed to it in mining and milling operations. The roentgen findings are similar to those seen in asbestos workers. Pleural plaques, diffuse pleural thickening, pleural calcification, and pleural effusion may be observed. Pulmonary parenchymal findings similar to those of asbestosis may also be observed. When talc contains silica, the findings resemble those of silicosis and may be simple, with small nodules, or complicated, with progressive massive pulmonary fibrosis. Emphysema is also a prominent feature of this disease.
A form of talcosis has been described in methadone abusers who inject oral methadone intravenously. Oral methadone contains talc, which appears to be the cause of a diffuse interstitial micronodular process in the lungs. This may progress to conglomerate upper-lobe, mass-like opacities similar to those of progressive massive fibrosis in silicosis. The masses may develop rapidly and are associated with basal emphysema and upper-lobe volume loss82. The diagnosis, as in the other pneumoconioses, is based on correlation of historical, clinical, and radiographic findings (Fig. 27-12).
RARE SILICATE PNEUMOCONIOSES