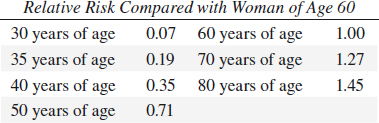• Ashkenazi Jewish women + nuns
• upper > lower social class
• unmarried > married women
• Whites > Blacks after age 40
REPRODUCTIVE VARIABLES in BREAST CANCER
• nulliparous > parous:
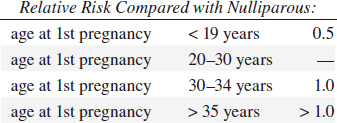
• first full-term pregnancy after age 35: 2 x risk
• low parity > high parity
• early age at menarche (< 12 years):
relative risk compared with onset of regular ovulatory cycle:
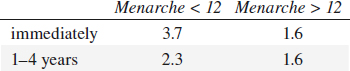
• late age at menopause:
relative risk compared with menopause before age 44:
natural menopause > 55 years of age 2.00
• early bilateral oophorectomy:
relative risk compared with menopause between ages 45–49 years:
artificial menopause at 50–54 years 1.34
artificial menopause before age 45 0.77
MULTIPLE PRIMARY CANCERS in BREAST CANCER
• 4–5 x increase in risk for cancer in contralateral breast
• increased risk after ovarian + endometrial cancer
BRCA (breast cancer)
= mutation of tumor suppressor gene accounts for 50% of hereditary breast cancers
5–10% of all breast cancers are hereditary!
• BRCA1 on long arm of chromosome 17
• BRCA2 on chromosome 13
Carrier: group of women at high risk for breast cancer
◊ Strict surveillance mandatory!
1% of women are at very high risk for developing breast cancer due to a genetic disposition!
FAMILY HISTORY of BREAST CANCER
• breast cancer in first-degree relative:
Relative risk compared with negative family Hx:
(+) for mother 1.8
(+) for sister 2.5
(+) for mother + sister 5.6
• 25% of patients with carcinoma have a positive family history
• carcinoma tends to affect successive generations ~ 10 years earlier
BENIGN BREAST DISEASE AND BREAST CANCER
• 2–4 x increased risk with atypical hyperplasia relative risk compared with no biopsy:
benign breast disease in all patients 1.5
nonproliferative disease 0.9
proliferative disease without atypia 1.6
fibroadenoma + hyperplasia 3.5
atypical duct hyperplasia (ADH):
no family history of breast cancer 4.4
family history of breast cancer 8.9
PARENCHYMAL BREAST PATTERN AND BREAST CANCER
• prominent duct pattern + extremely dense breasts according to Wolfe classification
N1 (0.14%), P1 (0.52%), P2 (1.95%), DY (5.22%)
RADIATION EXPOSURE and BREAST CANCER
excess risk of 3.5–6 cases per 1,000,000 women per year per rad after a minimum latent period of 10 years (atomic bomb, fluoroscopy during treatment of tuberculosis, irradiation for postpartum mastitis, Hodgkin disease)
GEOGRAPHY
• Western + industrialized nations (highest incidence)
• Asia, Latin America, Africa (decreased risk)
Breast Cancer Evaluation
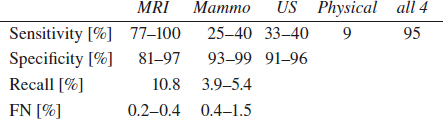
Localizing Signs of Breast Cancer
= PRIMARY SIGNS OF BREAST CANCER
1. Dominant mass seen on two views with
(a) Spiculation = stellate / star-burst appearance (= fine linear strands of tumor extension + desmoplastic response); “scirrhus” caused by:
(1) infiltrating ductal carcinoma (75% of all invasive cancers)
(2) invasive lobular carcinoma (occasionally)
√ mass feels larger than its mammographic / sonographic size
DDx: prior biopsy / trauma / infection
(b) Smooth border
(1) intracystic carcinoma (rare): subareolar area; bloody aspiration
(2) medullary carcinoma: soft tumor
(3) mucinous / colloid carcinoma: soft tumor
(4) papillary carcinoma
√ “telltale” signs: lobulation, small comet tail, flattening of one side of the lesion, slight irregularity
√ “halo” sign (= Mach band) may be present
DDx: cyst (sonographic evaluation)
(c) Lobulation
appearance similar to fibroadenoma (only characteristic calcifications may exclude malignancy)
◊ The likelihood of malignancy increases with number of lobulations
• clinical size of mass > radiographic size (1951) (Leborgne’s law)
[Raul Alfredo Leborgne Fossemale (1907–1986), Uruguayan radiologist created the mammograph, discovered radiological signs and developed the first method of radiologically guided mammary biopsy]
2. Asymmetric density = star-shaped lesion
√ distinct central tumor mass with volumetric rather than planar appearance → additional coned compression view
√ denser relative to other areas (= vessels + trabeculae cannot be seen within high-density lesion)
√ fat does not traverse density
√ corona of spicules
√ in any quadrant → but fatty replacement occurs last in upper outer quadrant
DDx: postsurgical fibrosis, traumatic fat necrosis, sclerosing duct hyperplasia
3. Microcalcifications
associated with malignant mass by mammogram in 40%, pathologically with special stains in 60%, on specimen radiography in 86%
◊ 20% of clustered microcalcifications represent a malignant process!
(a) shape: fragmented, irregular contour, polymorphic, casting rod-shaped without polarity, Y-shaped branching pattern, granular “salt and pepper” pattern, reticular pattern
(b) density: various densities
(c) size: 100–300 µm (usually); rarely up to 2 mm
(d) distribution: tight cluster over an area of 1 cm2 or less is most suggestive; coursing along ductal system seen in ductal carcinoma with comedo elements
4. Architectural distortion
Cause: desmoplastic reaction
√ ragged irregular borders
DDx: postsurgical fibrosis
5. Interval change
(a) neodensity = de novo developing density → in 6% malignant
(b) enlarging mass → in 10–15% malignant
6. Enlarged single duct
= low probability for cancer in asymptomatic woman with normal breast palpation
√ solitary dilated duct > 3 cm long
DDx: inspissated debris / blood / papilloma
7. Diffuse increase in density (late finding)
| Cause: | (1) | plugging of dermal lymphatics with tumor cells |
| (2) | less flattening of sclerotic + fibrous elements of neoplasm in comparison with more compressible fibroglandular breast tissue |
Nonlocalizing Signs of Breast Cancer
= SECONDARY SIGNS OF BREAST CANCER
1. Asymmetric thickening
2. Asymmetric ducts especially if discontinuous with subareolar area
3. Skin changes
(a) skin retraction = dimpling of skin
Cause: desmoplastic reaction → shortening of Cooper ligaments / direct extension of tumor to skin
DDx: trauma, biopsy, abscess, burns
(b) skin thickening ← blocked lymphatic drainage / tumor in lymphatics
• peau d’orange
DDx: normal in inframammary region
4. Nipple / areolar abnormalities
(a) retraction / flattening of nipple
DDx: normal variant
(b) Paget disease = eczematoid appearance of nipple + areola in ductal carcinoma
√ associated with ductal calcifications toward nipple
DDx: nipple eczema
(c) nipple discharge
• spontaneous persistent discharge
• need not be bloody
DDx: lactational discharge
5. Axillary nodes (sign of advanced / occult cancer)
√ > 1.5 cm without fatty center
DDx: reactive hyperplasia
6. Abnormal veins
√ venous diameter ratio of > 1.4÷1 in 75% of cancers (= late sign + thus not very important)
Location of Breast Masses
benign + malignant masses are of similar distribution
@ upper outer quadrant 54%
@ upper inner quadrant 14%
@ lower outer quadrant 10%
@ lower inner quadrant 7%
@ retroareolar 15%
◊ Mediolateral oblique view is important part of screening because it includes largest portion of breast tissue + considers most common location of cancers!
Metastatic Breast Cancer
@ Axillary lymph adenopathy
Prevalence: 40–74%
Risk for positive nodes: 30% if primary > 1 cm, 15% if primary < 1 cm
@ Bone
@ Liver
Prevalence: 48–60%
US:
√ hypoechoic (83%) / hyperechoic (17%) mass
Screening of Asymptomatic Patients
Definition of screening (World Health Organization):
A screening test must
(a) be adequately sensitive and specific
(b) be reproducible in its results
(c) identify previously undiagnosed disease
(d) be affordable
(e) be acceptable to the public
(f) include follow-up services
Guidelines of American Cancer Society, American College of Radiology, American Medical Association, National Cancer Institute:
1. Breast self-examination to begin at age 20
2. Breast examination by physician every 3 years between 20–40 years, in yearly intervals after age 40
3. Baseline mammogram between age 35–40; follow-up screening based upon parenchymal pattern + family Hx
4. Initial screening at 30 years if patient has first-degree relative with breast cancer in premenopausal years; follow-up screening based upon parenchymal pattern
5. Mammography at yearly intervals after age 40
6. All women who have had prior breast cancer require annual follow-up
Additional recommendations:
7. Screening at 2-year intervals for women > 70 years
8. Baseline mammogram 10 years earlier than age of mother / sister when their cancer was diagnosed
Rate of detected abnormalities:
30 abnormalities in 1,000 screening mammograms:
20–23 benign lesions
7–10 cancers
Acceptable recall rate for screening examination:
10% for initial prevalence screening;
5% for subsequent incidence screening
Interval cancers: 10–20% of cancers surface between annual screenings
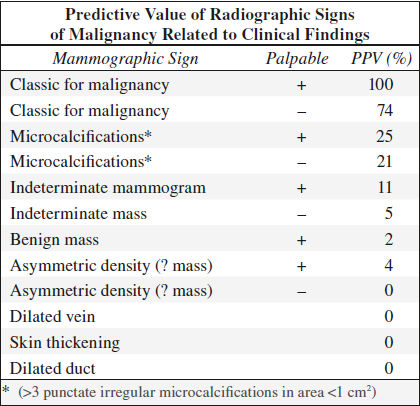
Role of Mammography
Overall detection rate:
58–69%; 8% if < 1 cm in size
Mammographic accuracy:
| 88% | correctly diagnosed by radiologist |
| 27% | detected only by mammography |
| 8% | misinterpretations |
| 4% | not detected |
| 15–30% | positive predictive value (national average): 25% PPV for women in 5th decade 50% PPV for women in 8th decade |
Value of Screening Mammography
Indication:
decrease in cancer mortality through earlier detection + intervention when tumor size is small + lymph nodes negative; tumor grade of no prognostic significance in tumors < 10 mm in size
1. Health Insurance Plan (HIP) 1963–1969
randomized controlled study of 62,000 women aged 40–64
› 25–30% reduction in mortality in women > 50 years (followed for 18 years)
› 25% reduction in mortality in women 40–49 years (followed for 18 years); no significant effect at 5- and 10-year follow-up
› 19% of cancers found by mammography alone
› 61% of cancers found at physical examination
› effectiveness of screening < 50 years of age is uncertain
2. Breast Cancer Detection Demonstration Project (BCDDP) 1973–1980
4,443 cancers found in 283,000 asymptomatic volunteers
› 41.6% of cancers found by mammography alone (77% with negative nodes)
› 8.7% of cancers found by physical examination alone
› 59% of noninfiltrating cancers found by mammography alone
› 25% of cancers were intraductal (versus 5% in previous series)
› 21% of cancers found in women aged 40–49 years (mammography alone detected 35.4%)
› 51% of cancers found with both mammography + physical examination
3. Swedish Two-county Trial 1977–1990
randomized controlled study of 78,000 women in study group + 56,700 in control group aged 40–74 years with screening phase lasting 7 years
(a) single MLO mammogram at 2-year intervals for women < 50 years of age
(b) single MLO mammogram at 3-year intervals for women ≥ 50 years of age
› 40% reduction in mortality at 7 years in women 50–74 years
› 0% reduction in mortality at 7 years in women 40–49 years
› At 29-year follow-up 34 years of life per 1000 women were saved screened over a 7-year period
› At 29-year follow-up 1 breast cancer was prevented for each 519 women screened for 7 years
4. Meta-analysis of combined results of 5 Swedish trials for women aged 39–49
• 29% reduction in breast cancer mortality with screening mammograms offered at intervals from 18 to 28 months
OCCULT VERSUS PALPABLE BREAST CANCER
◊ 27% are occult cancers (NO age difference)
Positive axillary nodes:
occult cancers (19%); palpable cancers (44%)
10-year survival:
occult cancers (65%); palpable cancers (25%)
Mammographically Missed Cancers
False-negative screening mammogram:
= pathologic diagnosis of breast cancer within 1 year after negative mammogram with the following types of misses:
(a) lesion could not be seen in retrospect (25–33%) = “acute cancer” = cancer surfacing in screening interval
(b) cancer undetected by first reader but correctly identified by second reader (14%)
(c) cancer visible in retrospect on prior mammogram (61%)
Prevalence: ~ 4–15–34% of all cancers; ~ 3 cancers ÷ 2,000 mammograms; 5–15–22% of palpable breast cancers
◊ A second reader will detect an additional 5–15% of cancers!
Cause:
1. Logistical error
(a) prior films not made available
(a) prior films not back far enough
◊ For comparison use a mammographic interval of 3 years to detect slowly developing asymmetries (tumor doubling time ~150 days)
2. Interpretation error (52%):
(a) benign appearance (18%): medullary carcinoma, colloid carcinoma, intracystic papillary carcinoma, some infiltrating ductal carcinomas
(b) present on previous mammogram (17%)
(c) seen on one view only (9%)
(d) site of previous biopsy (8%)
(e) calcifications assumed to be benign
3. Observer error (30–43%):
(a) overlooked: one lesion / group of calcifications doesn’t fit the rule of multiplicity
(b) intrinsic distraction = presence of an obvious finding leads to overlooking of a more subtle lesion = “satisfied search” phenomenon
(c) no knowledge of clinical finding
(d) rushed interpretation
(f) extraneous distraction
(g) eye fatigue
(h) inexperience
4. Wrong assumption
(a) misleading long history of a lump results in screening rather than diagnostic mammogram
(b) acceptance of irreconcilable size discrepancy / lesion location between sonogram and mammogram
(c) suspicious findings remain suspicious!
4. Technical error (5%):
(a) inadequate radiographic technique: improper positioning, inadequate compression, under- / overexposed image, poor screen-film contact, geometric motion blurring
(b) misapplied workup = failure to image region of interest: spot compression magnification view may displace lesion out of field of view
(c) suboptimal viewing conditions: inadequate luminance of view boxes, extraneous view box light, high ambient room light
5. Tumor biology:
(a) small tumor size
(b) failure to incite desmoplastic reaction
(eg, invasive lobular carcinoma)
(c) limitations of screen-film mammography in physically dense breasts
(d) no associated microcalcifications (~ 50% of cancers)
(e) developing soft-tissue density
◊ A developing density that cannot be reconciled with a history of trauma, inflammation or hormones and does not represent a cyst or lymph node requires a biopsy!
(f) stability of mammographic findings
◊ Malignant calcifications may be stable for up to 63 months
◊ A mass may not change for up to 4.5 years
6. Difficult location:
(a) axillary tail
(b) inframammary fold
(c) busy subareolar region
Location of missed cancers:
retroglandular area (33%), lateral parenchyma (31%), central (18%), medial (13%), subareolar (4%)
DISCLOSING UNANTICIPATED OUTCOMES
(a) Content to be disclosed to patient:
1. Provide facts about events = presence of error / system failure if known
2. Express regret = apology
(b) Institutional requirements:
1. Integrate disclosure, patient safety and risk management
2. Establish disclosure support system
3. Use performance improvement tools to track and enhance disclosure
Radiation-induced Breast Carcinoma
◊ Lifetime risk with cumulative carcinogenic effect related to age!
(a) women age < 35: 7.5 additional cancers per 1 million irradiated women per year per rad
(b) women age > 35: 3.5 additional cancers per 1 million irradiated women per year per rad
Role of Breast Ultrasound in Breast Cancer
Indications:
◊ Ultrasound is no screening tool!
A. TARGETED EXAM
(1) Initial study of palpable lump in patient < 30 years of age / pregnant / lactating
◊ Ultrasound will not add useful information in an area that contains only fatty tissue on a mammogram!
(2) Characterization of mammographic / palpable mass as fluid-filled / solid
◊ Ultrasound will add useful information if there is water-density tissue in the area of palpable abnormality!
◊ Differentiation of cystic from solid lesion is the principal role of ultrasound!
(3) Additional evaluation of nonpalpable abnormality with uncertain mammographic diagnosis
(4) Search for focal lesion as cause for mammographic asymmetric density
(5) Confirmation of lesion seen in one mammographic projection only
B. WHOLE-BREAST EXAM
(1) Breast secretions
(2) Suspected leaks from silicone implant
(3) Follow-up of multiple known mammographic / sonographic lesions
(4) Radiographically dense breast with strong family history of breast cancer
(5) Metastases thought to be of breast origin, but with negative clinical + mammographic exam
(6) Mammography not possible: “radiophobic” patient, bedridden patient, after mastectomy
C. INTERVENTIONAL PROCEDURE
(1) Ultrasound-guided cyst aspiration
(2) Ultrasound-guided core biopsy
(3) Ultrasound-guided ductography, if
(a) secretions cannot be expressed
(b) duct cannot be cannulated
Accuracy: 98% accuracy for cysts; 99% accuracy for solid masses; small carcinomas have the least characteristic features
Role of Breast MR in Breast Cancer
◊ Impact of routine breast MRI on survival still unknown → use of MRI for preoperative staging controversial!
Pathophysiology of tumor detection:
growth of tumor vessels (= neoangiogenesis) with abnormal leaky capillaries (= increased permeability) → rapid uptake (= early enhancement) and rapid washout of intravenous contrast material in cancerous lesions
Indications:
(1) Improve detection + characterization of primary
• palpable mass + negative mammogram + sonogram
• status post lumpectomy with positive resection margins
◊ Helps to reduce the rate of re-excision (4–43%)
• repeated indeterminate mammogram
• staging:
› tumor size: MR more accurate in estimate of tumor size than mammography / ultrasound
› detection of extensive intraductal component: MR superior to mammography
› multifocality (16–50%): in 70% detected by MR only
› multicentricity(15–30%): in 50% detected by MR only
◊ Reduces rate of “recurrence” / preoperatively undetected foci of cancer from 6.8% to 1.2%
◊ May change accelerated partial breast irradiation to whole-breast irradiation
› before + after 2nd cycle of neoadjuvant chemotherapy to separate responders from nonresponders
› chest wall invasion
(2) “Clearing” (= screening) of contralateral breast:
bilaterality (3–5%) in 75% detected by MR only
◊ Increases detection of contralateral breast cancer from 4% to 17% (of which 35% are DCIS)
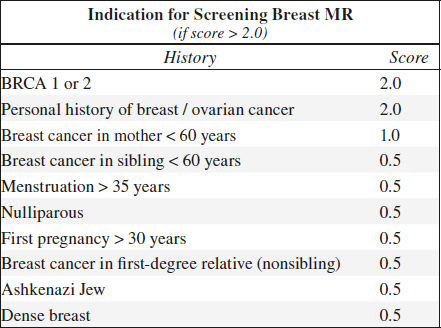
(3) Screening of high-risk groups
• young patient with positive BRCA1 gene
• dense breast + high-risk lesion of LCIS
• status post mastectomy + breast reconstruction with implant (yearly screening)
(4) Improve detection of recurrent breast cancer
• planning for biopsy to determine scar versus recurrent tumor after breast-conserving therapy
(5) Evaluate response to neoadjuvant chemotherapy
(6) Examine breasts in patient with metastatic breast cancer but unknown origin of primary (2–7%)
• axillary node malignancy + negative mammogram
(7) Implant imaging
Sensitivity: 72–90–100% (for DCIS 40–73–100%, for invasive cancer 91%)
Specificity: 37–70–100%
◊ A normal MR mammogram
› correctly rules out malignancy in > 96%
› means no invasive cancer > 3 mm
› means no further exam for 2 years (for 1 year if BRCA positive)
› FN: DCIS, LCIS, lobular cancer, tubular cancer
Optimal timing of MR: 7–20 days after beginning of cycle; 6 months after open biopsy; 12 months after radiation therapy
MR:
Malignant morphology always trumps kinetics!
√ reduced signal on T2WI
√ irregular morphology
√ lymphangitic bridges / streaks
√ contrast enhancement:
√ rapid ⇑ in SI after contrast injection = rapid wash-in
◊ 90/90 rule = cancers show an SI increase of > 90% in the first 90 sec!
√ markedly ⇑ amplitude than normal parenchymal tissue
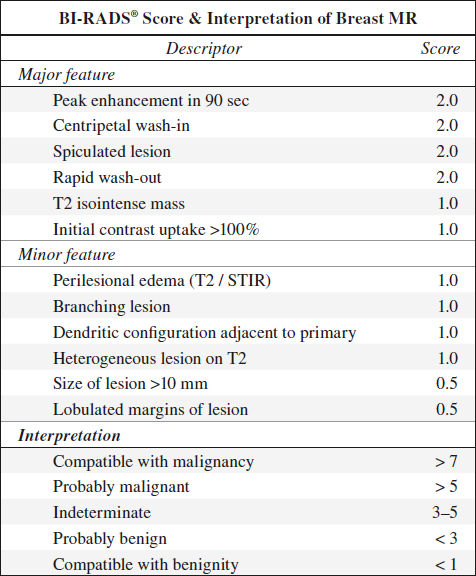
√ plateau / fast wash-out in postinitial phase
√ “arterial feeder” sign
√ intense early rim / peripheral enhancement (± central necrosis)
√ centripetal progression of enhancement
√ malignant mass margination
Slowly / Nonenhancing Breast Cancer on MR
1. Lobular carcinoma
2. Tubular carcinoma
3. Mucinous carcinoma
4. Grade I invasive ductal carcinoma
Role of PET/CT in Breast Cancer
PET-CT useful ONLY in
(a) inflammatory breast cancer
(b) > 3 cm large breast cancer
(c) stage II/III breast cancer
Staging of Breast Cancer
(1) Initial staging
◊ PET/CT is efficient for locally advanced + inflammatory breast cancer
√ 40% of breast primaries NOT visualized
√ 61% sensitive + 80% specific for axillary nodes
√ unsuspected disease detected in mediastinal / internal mammary lymph nodes in 30%
(2) Restaging
◊ PET/CT performs better than all other imaging
√ 92–100% sensitive for recurrence, 72–82% specific (changes with time interval since therapy)
√ PET/CT may change clinical management in 36%
High SUV Values in Breast Cancer
(1) Ranking: infiltrating ductal carcinoma > infiltrating lobular carcinoma > ductal carcinoma in situ
(2) Estrogen receptor-negative tumor
(3) Cancer with poor prognosis: triple negative breast tumor (negative for estrogen + progesterone receptors + HER2/neu overexpression)
Distant Metastases in Breast Cancer
Soft-tissue lesion: mediastinal nodes (24%), liver (15%), supracentimetric lung nodules
Bone lesion:
(a) osteosclerotic: PET lacks sensitivity; downstaging with no FDG uptake (in 12%)
(b) osteolytic / mixed lesions: PET more efficient than CT / bone scintigraphy
◊ Bone scintigraphy may no longer be necessary!
Role of Stereotactic Biopsy
Indications: obviously malignant nonpalpable lesion, indeterminate likely benign lesion, anxiety over lesion
Targets: well-defined solid mass, indistinct / spiculated mass, clustered microcalcifications
Advantage: single-stage surgical procedure
Problematic: 3–5-mm small lesion, fine scattered microcalcifications, indistinct density, area of architectural distortion
Sensitivity: 85–99% with core needle biopsy (100% specific), 68–93% with fine-needle aspiration (88–100% specific)
Indication for excision:
(a) anatomic reason: lesion close to chest wall, lesion in axillary tail, very superficial lesion
(b) pathologic reason:
1. Radial scar suspected (in up to 28% associated with tubular carcinoma)
2. Atypia / atypical hyperplasia (in 49–61% associated with malignancy)
3. Carcinoma in situ (in 9–20% associated with invasion)
4. Branching microcalcifications suggestive of DCIS with comedo necrosis
Miss rate: 3–8% for stereotactic biopsy, 3% for surgery
Incidental Breast Cancer Detection by CT
› highly predictive features:
√ irregular margins, irregular shape, rim enhancement
√ washout pattern on postcontrast images
√ diffuse regional enhancement
› most accurate sign:
√ spiculated + irregular margin
Categories of Breast Cancer
A. Invasive ductal carcinoma (NOS) + DCIS 85%
B. Other types of malignancy 15%
(a) Invasive lobular carcinoma (ILC)
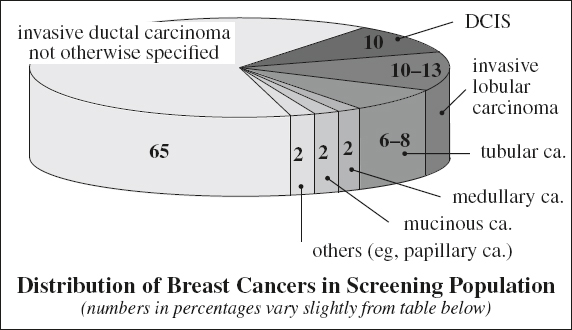
(b) Well-differentiated subtypes of IDC
1. Tubular carcinoma
2. Mucinous carcinoma
3. Medullary carcinoma
4. Papillary carcinoma
(c) Cancers of stromal origin
1. Phyllodes tumor
2. Angiosarcoma
3. Osteosarcoma
4. Adenoid cystic carcinoma
(d) Metastatic disease (0.5–2.0%)
1. NHL
2. Malignant melanoma
3. Metastatic carcinoma
4. Rhabdomyosarcoma
5. Leukemia
Origin: terminal ductal lobular unit (TDLU);
| terminal duct | → | ductal carcinoma (85%) |
| acinus | → | lobular carcinoma (10–12%) |
| stroma | → | fibrosarcoma, liposarcoma, angiosarcoma, phylloides sarcoma |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree