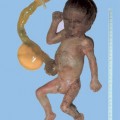
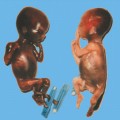
18 Drugs Substances: Phenytoin, carbamazepine, valproic acid, phenobarbiturate. Definition: Effects of anticonvulsive drugs with a known teratogenic effect on the fetus. In the presence of epilepsy, there is a two- to threefold increase in the risk of fetal anomalies, even without the effects of medication. Incidence: The risk of fetal malformations lies at 10% if medications are given in early pregnancy. In patients with an autosomal-recessively inherited deficiency in epoxide hydrolase (a metabolic enzyme), the risk of fetal anomalies under the influence of anticonvulsive drugs is extremely high. Associated malformations: Phenytoin and carbamazepine may cause facial dysmorphism, microcephaly, finger hypoplasia, nail hypoplasia, growth restriction, and mental impairment. Valproic acid and carbamazepine increase the risk of neural tube defects to 1%. Clinical features: Central nervous system anomalies: microcephaly, holoprosencephaly, myelomeningocele. Facial anomalies: hypertelorism, cleft lip and palate, stubby nose, wide nasal bridge. Skeletal anomalies: hypoplasia of the distal phalanges, ulnar deviation of the thumb, hip dysplasia, short neck, pterygium of the neck, anomalies of the ribs and sternum. Cardiac malformations: VSD, pulmonary stenosis, aortic stenosis. Anomalies of the urogenital system: ambiguous genitalia, renal anomalies. Growth restriction and oligohydramnios. Clinical management: Karyotyping (differential diagnosis). Measurement of epoxide hydrolase in amniotic fluid; this is still experimental. There is an increased risk of cranial bleeding if medications belonging to the hydantoin group are used. In this situation, vitamin K should be administered to the mother before the start of labor. Procedure after birth: When the mother is receiving hydantoin, vitamin K should be given. Intracranial bleeding should be excluded. With carbamazepine, growth restriction and developmental disturbances are frequently expected at the neonatal stage. Prognosis: Over 90% of women suffering from fits have normal children. The prognosis depends on the type and severity of fetal malformation. Self-Help Organization Title: Epilepsy Foundation Description: Information and support for people with epilepsy, their families and friends. Pharmaceutical program, newsletter for members. Affiliates’ development kit. Referrals to local affiliates (many of which have employment-related programs). Information and referrals. Scope: National (English/Spanish) Number of groups: 60 + affiliates Founded: 1967 Address: 4351 Garden City Dr., Landover, MD 20785, United States Telephone: Information 800–332–1000; library 800–332–4050; catalog sales 800–213–5821; 301–459–3700 Fax: 301–577–4941 E-mail: postmaster@efa.org Buehler BA, Delimont D, van Waes M, Finnell RH. Prenatal prediction of risk of the fetal hydantoin syndrome. N Engl J Med 1990; 322: 1567–72. Ceci O, Loizzi P, Caruso G, Caradonna F, Clemente R, Ferreri R. Fetal malformations in an epileptic pregnant woman treated with carbamazepine. Zentralbl Gynäkol 1996; 118: 169–71. Dean JC, Hailey H, Moore SJ, Lloyd DJ, Turnpenny PD, Little J. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J Med Genet 2002; 39: 251–9. Giardina S, Contarini A, Becca B. Maternal diseases and congenital malformations. Ann Ist Super Sanita 1993; 29: 69–76. Holmes LB. Looking for long-term effects from prenatal exposures to anticonvulsants. Teratology 2001; 64: 175–6. Janz D. Pregnancy and fetal development in epileptic women. Geburtshilfe Frauenheilkd 1984; 44: 428–34. Koch S, Losche G, Jager R, et al. Major and minor birth malformations and antiepileptic drugs. Neurology 1992; 42: 83–8. Kroes HY, Reefhuis J, Cornel MC. Is there an association between maternal carbamazepine use during pregnancy and eye malformations in the child? [review]. Epilepsia 2002; 43: 929–31. Lowe SA. Drugs in pregnancy: anticonvulsants and drugs for neurological disease [review]. Best Pract Res Clin Obstet Gynaecol 2001; 15: 863–76. McAuley JW, Anderson GD. Treatment of epilepsy in women of reproductive age: pharmacokinetic considerations [review]. Clin Pharmacokinet 2002; 41: 559–79. Olafsson E, Hallgrimsson JT, Hauser WA, Ludvigsson P, Gudmundsson G. Pregnancies of women with epilepsy: a population-based study in Iceland. Epilepsia 1998; 39: 887–92. Omtzigt JG, Los FJ, Grobbee DE, et al. The risk of spina bi-fida aperta after first-trimester exposure to valproate in a prenatal cohort. Neurology 1992; 42: 119–25. Samrén EB, van Duijn CM, Koch S et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia 1997; 38: 981–90.
Anticonvulsive Drugs
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree