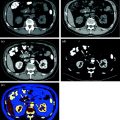
Fig. 2.1
Graph shows iodine photoabsorption (blue), Compton effect (green), and coherent (Rayleigh) scattering (red) at different energies. Note that photoabsorption effect of iodine is maximized at 50 keV (at a mean photon energy of 80 kVp). Maximum photoabsorption is shown slightly above iodine’s K-edge (33 keV)
Compton scattering [15] occurs when photons interact with outermost valence electrons, when the incident photon energy exceeds the binding energy of the free electron, that is ejected [16].
Photoelectric effect is the ejection of an electron from the innermost shell of an atom (K shell) by an incident photon. The void is filled from an adjacent shell causing energy release in the form of a photoelectron. The K–shell binding energy varies for each element and is directly proportional to the atomic number [16].
The K–edge is the spike in attenuation occurring at energy levels greater than that of the K-shell binding. K-edge values vary for each element and increase according to the atomic number. Photoelectric effect and K-edge behavior are the basis of DECT technique [2].
Iodine (I)-based compounds are the preferred contrast media in computed tomography. The relation between iodine (Z = 53) and its K-edge has been investigated [17, 18] and is set at 33 keV. The Iodine K-edge characteristics may be exploited in dual-energy and also low-kVp mono-energetic CT protocols [19–25]. The advantage is the enhanced conspicuity of iodine and a relative dose reduction. In this regard, we acknowledge that kVp and effective radiation dose share an exponential relation where mAs and dose a linear one [26]. As a drawback, a high-tube-current (mAs) setting is technically necessary to decrease image rumor [23].
Material differentiation, including iodine, derived from a multi-energy approach is at the basis of various DECT clinical applications (Fig. 2.2).
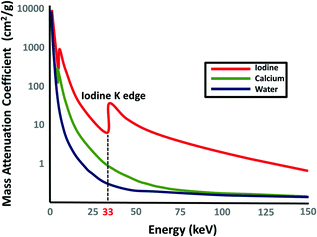

Fig. 2.2
Graph showing mass-attenuation coefficients for iodine (red), calcium (green), and water (blue) at different energies. Note the attenuation peak of iodine (Z = 53) at 33 keV
2.3 DECT and Iodinated CM in Clinical Practice
Material differentiation and contrast material quantification–identification are based upon the previously described basic technical principles, making the DECT technique highly attractive.
Material differentiation, considered as the ability to distinguish different materials, including iodine [1] may be exploited in different ways.
It has been extensively investigated in vascular imaging, in which a DECT protocol in the high- and low-energy spectra has been proven useful in aortic and visceral imaging, distal runoffs, and cervical CT angiography [9].
In aortic imaging, iodine-selective maps may highlight differences between high-attenuating blood, high-attenuating bone, and iodine increasing diagnostic confidence in the evaluation of subtle endoleaks [27]. Also the ability of generating post-processed bone-subtracted three-dimensional aortic CTA images has been proven useful in clarifying complex vascular anatomy and related abnormalities [28].
In peripheral CTA, bone subtraction using a DECT approach has been shown to be a superior and faster option against conventional bone segmentation [29, 30]; unfortunately, incomplete subtraction and vascular truncation may occur [31]. Despite that, in comparison to DSA, a dual-energy bone-subtracted peripheral CTA is highly sensitive, specific, and accurate (94–97 %) [32]. Calcific plaque removal may also be an advantageous application, although has been observed to better perform in the aorto-iliac tract rather than distal segments [33]. It has been demonstrated that also highly calcified plaques may be removed from vessel walls at post-processing both in phantom [34] and in vivo [35] studies.
In the evaluation of intracranial vessels, DECT angiography with bone subtraction appears to be more accurate and faster than threshold-based bone subtraction. Residual bone and vessel truncation is usually present with the threshold-based method, due to narrow space between enhancing vessels and bony structure [36]. In comparison to mask-subtracted CTA, DECT proved to deliver similar diagnostic results [37]. Calcified plaque removal and characterization using DECT has been also demonstrated in the intracranial vascular evaluation with promising results [38–41].
Relatively to contrast material differentiation and quantification is acknowledged that iodine attenuation increases approximately by a twofold factor at 80 kVp compared with 140 kVp. Therefore, an increase in iodine conspicuity may be observed at lower-kVp imaging both in dual-energy studies and low-kVp mono-energetic acquisitions [23]. This concept has been applied both to hypervascular [22] and hypovascular lesions [42] in the liver, considering for the latter the advantage of a higher lesion-background contrast difference.
The ability of selectively discriminating iodine allows its subtraction and the eventual display of its distribution. Therefore, virtual non-contrast imaging is another feasible clinical application of DECT that allows selective iodine subtraction [43, 44] and also dose reduction [45].
Virtual non-contrast imaging is an algorithm-based subtraction technique [46]. Virtual images have been proven an acceptable substitute of standard non-enhanced pre-contrast imaging [44], with a dose decrease up to 50 % [47]. Clinical application and usefulness has been demonstrated both in abdominal [7] and vascular imaging [9] (Fig. 2.3).
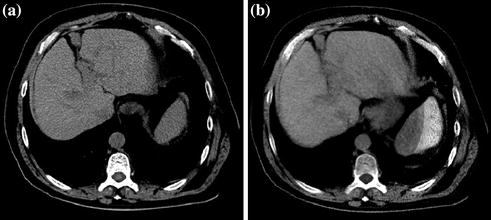

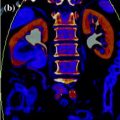
Fig. 2.3
Pre-contrast (a) and corresponding virtual non-contrast image (b) of the liver
Iodine color-coded imaging and iodine perfusion maps follow the same principle and allow quantitative display of iodine concentration (Fig. 2.4). These applications have been proven useful in genitourinary [47–49], thoracic [50, 51], and cardiovascular [52, 53] imaging.
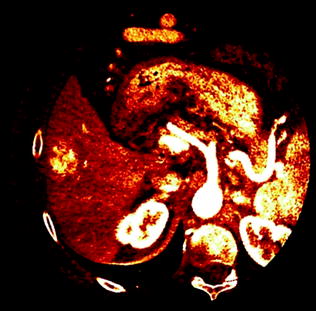

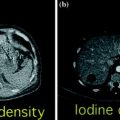
Fig. 2.4
Color-coded Iodine distribution map derived from a DECT study of the liver. Note the right lobe hypervascular liver lesion along with the exquisite overall vascular depiction
In aortic imaging, advantages of dual-spectra acquisitions may be applied to improve poorly opacified studies and enhance visualization of small vascular branches. The lower energy acquisition may aid in recognition of subtle endoleaks in the perigraft space after repair [20, 54, 55]. In general, we suggest evaluating both energy acquisitions along with blended images in order to enhance diagnostic accuracy, during DECT studies reading (Fig. 2.5).
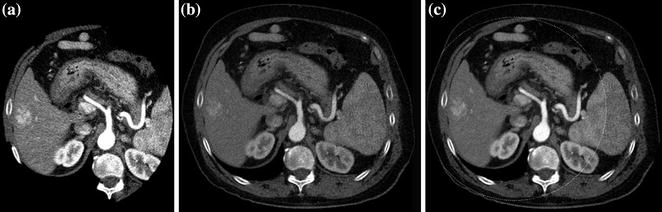

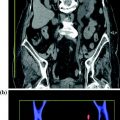
Fig. 2.5
Different iodine conspicuities demonstrated at 80 kVp (a) and 140 kVp (b) in a DECT liver study. A blended image (0.3 factor) is also presented (c). Note the augmented conspicuity of iodine in the lower-energy acquisition (a)
It has been demonstrated that endoleak attenuation is higher at lower energies [55]. A single DECT-delayed acquisition has been proposed as a possible approach in post-treatment EVAR evaluation, with the advantage of dose reduction [56, 57].
In aortic CT angiography, the standard CT protocol includes unenhanced images to detect intramural hematoma or vessel calcifications especially in dissections and performance of virtual non-contrast imaging from a DECT protocol has been consequently evaluated. In this setting, it has been confirmed that a virtual unenhanced reconstruction may substitute standard unenhanced images and reduce the delivered dose [58].
2.4 Spectral Imaging and Iodine
Spectral CT is presently under investigation and only limited to research environments. It relies on photon-counting detector systems and the spectral information may be used to distinguish different materials based on their K-edge [53, 59, 60]. The identification of different K-edges allows the distinction of multiple high atomic numbers elements simultaneously, allowing multi-element K-edge imaging. Iodine (Z = 53; K-edge = 33.2 keV) is among those elements. Unfortunately, photon starvation is a serious problem in the lower-energy range; therefore, gadolinium (Z = 64; K-edge = 50.2 keV) appears to be a more promising detectable agent [60]. A preliminary study has been conducted in mice where the authors demonstrated simultaneous depiction of iodine, calcium, and barium [12].
Recent introduction of the Gemstone Spectral Imaging (GSI) system (GE, Milwaukee, USA) with broad keV manipulation appears also promising in terms of material differentiation (iodine-calcium-water) and iodine mapping [61].
2.5 Low-kVp Protocols and Iodine: Conspicuity and Dose Reduction
Based on the aforementioned CT physics principles, a mono-energetic low-kVp CT acquisition, near to the iodine K-edge (K-edge = 33.2 keV), is an advantageous “contrast-enhancing” strategy. Unfortunately, a mono-energetic low-kVp CT acquisition has the drawback of a higher noise and a high-tube-current setting is necessary to decrease it [23]. Clinical applications of mono-energetic low kVp have been described in vascular [28, 62, 63], abdominal [23, 64], and neck [25] contrast-enhanced CT protocols demonstrating both contrast-enhanced conspicuity and dose lowering.
In particular, Schindera et al. performed an in vitro experimental study evaluating depiction of simulated hypervascular lesions along with noise, contrast-to-noise ratio, lesion conspicuity, and dose for a low-voltage high-current CT protocol [65]. Their experiment included CT analysis of different concentration of iodinated solutions (from 4.0 to 5.4 mg I/mL) scanned at 140, 120, 100, and 80 kVp with an indirectly proportional mAs increment. Results of their study demonstrated 45 % of noise increase using an 80-kVp protocol compared to 140-kVp. Despite the noise increase, the lower-energy protocol had the highest contrast-to-noise ratio, the highest contrast conspicuity and the lowest delivered radiation dose, demonstrating potential advantages of eventually adopting such imaging strategy.
Recently, CT manufacturers are introducing iterative reconstruction (IR) techniques (ASIR, MBIR, IRIS, AIDR, iDose) to compensate for image noise when using lower tube voltage settings. A dose decreases up to 65 %, when using a low-kVp iteratively reconstructed abdominal CT protocol has been observed [23, 66–69].
Interestingly Cho and colleagues compared two different mono-energetic CT protocols at different energies (80 vs. 120 kVp) using two different iodinated contrast concentrations (300 vs. 370 mg/mL) for CT angiography of the renal arteries [63]. Results of their study indicated that using an 80-kVp protocol with moderately concentrated contrast media (300 mg/mL) might improve arterial enhancement delivering high image quality, using a smaller amount of iodine and lower radiation dose. The authors advocate the use of this imaging strategy to potentially reduce the risk of contrast-induced nephropathy in high-risk patients.
2.6 Conclusion
In conclusion, dual- and multi-energy computed tomographs are able to differentiate materials based on tissue analysis relatively to energy and matter interaction. To better understand the rationale behind material differentiation, thus iodinated contrast media behavior in multi-energy CT studies, the relation between tube kilovoltage and K-edge for iodine should always be recalled.
Latest generation tomographs are able to deliver a broad energy spectrum (kVp) and high power (mAs). Powerful hardware combined with novel software algorithms allows fascinating post-processing techniques such as selective subtraction imaging (virtual imaging) and iodine distribution mapping among others.
Dual-energy imaging is currently available in most advanced diagnostic centers while spectral imaging is still in an early and experimental phase.
Radiologists and clinicians should be familiar with DECT technical features and iodine-energy-related characteristics to exploit this novel technique in the clinical setting.
Mono-energetic low-kVp CT protocols may be also used to exploit iodine behavior in the lower-energy spectra.
References
1.
2.
3.
4.
5.
Kalender WA, Perman WH, Vetter JR, Klotz E (1986) Evaluation of a prototype dual-energy computed tomographic apparatus. I. Phantom Stud. Med Phys. 13(3):334–339CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree