Fig. 7.1
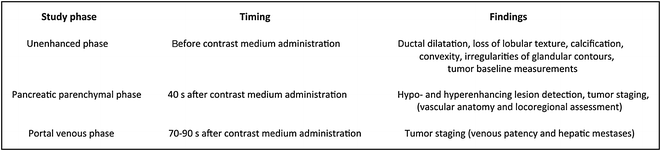
Intra-ductal papillary mucinous neoplasm of the pancreatic head. The lesion (arrow) appears slightly hypodense to normal pancreatic parenchyma on the unenhanced image (a) and is well identifiable on the contrast-enhanced pancreatic parenchymal phase image (b)
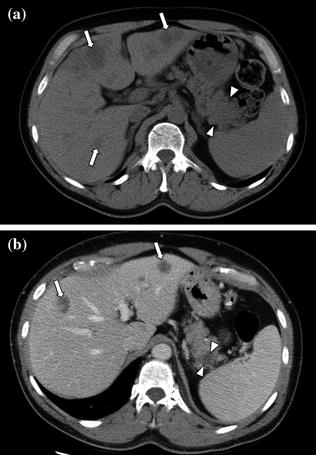
Fig. 7.2
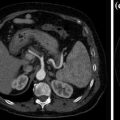
Adenocarcinoma of the pancreatic tail. An enlargement of the pancreatic tail, associated with loss of lobular texture, convexity, and irregularities of contours (arrowheads), is visible on the unenhanced image (a); multiple hepatic hypodense lesions (arrows) are also visible. Contrast-enhanced portal venous phase image (b) clearly shows a hypodense solid lesion (arrowheads) in the pancreatic tail with associated hepatic metastases (arrows)
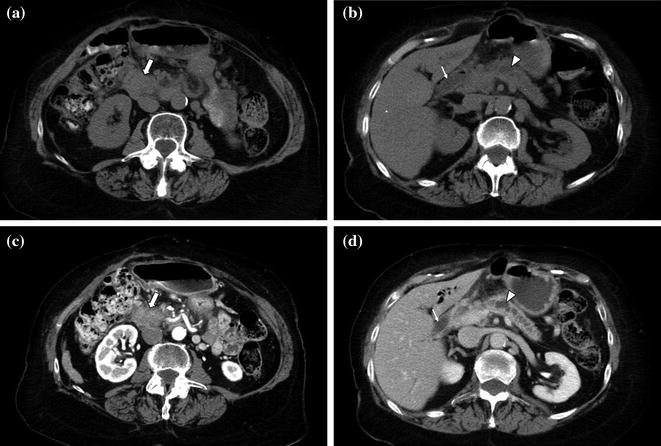
Fig. 7.3
Adenocarcinoma of the pancreatic head. Unenhanced (a, b), contrast-enhanced pancreatic parenchymal- (c), and portal venous (d) phase images show an enlargement of the inferior portion of the pancreatic head, with convexity and irregularities of contours (arrow), with dilatation of both the pancreatic (arrowhead) and common bile ducts (thin arrow) (“double duct sign”)
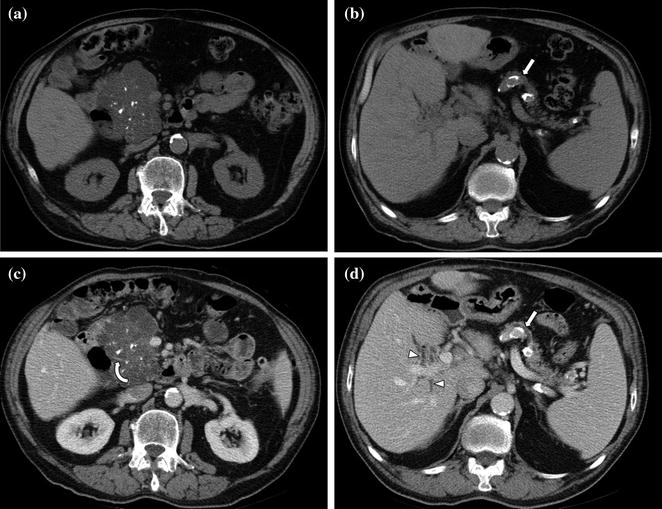
Fig. 7.4
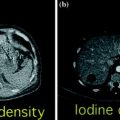
Serous cystadenoma of the pancreatic head. Unenhanced images (a, b) show a large multi-locular mass with multiple internal calcification within the pancreatic head (a), associated with upstream pancreatic main duct dilatation (arrow) and parenchymal atrophy (b). Contrast-enhanced portal venous phase images (c, d) better depict some enhancing septations (curved arrow) within the mass (c), upstream pancreatic main duct dilatation (arrow) as well as intra-hepatic biliary duct dilation (arrowheads) (d)
The administration of intravenous contrast material is a crucial requirement in MDCT protocols for pancreatic lesion evaluation. Contrast-enhanced CT has a sensitivity ranging from 89 to 97 % for tumor detection and a positive predictive value of 89–100 % for determining tumor unresectability [1, 8]. To maximize the conspicuity of vessels and pancreatic tumors, the pancreas and adjacent visceral structures are commonly scanned during different phases of contrast enhancement. In general, a biphasic CT study of the pancreas, consisting of pancreatic parenchymal and venous phase acquisitions, is currently the accepted technique for the detection and staging of pancreatic ductal adenocarcinoma (Fig. 7.5) [21–29]. Note that the pancreatic parenchymal phase occurs approximately 10–15 s later than the late hepatic arterial phase used for hypervascular liver lesion detection. In particular, with the use of modern bolus-tracking timing techniques, it starts with a delay of about 17–18 s after arrival of the bolus in the abdominal aorta at predefined attenuation threshold of 100 HU.
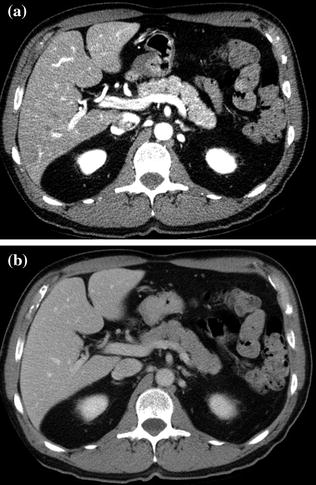

Fig. 7.5
Example of contrast-enhanced biphasic MDCT study of the pancreas, consisting of contrast-enhanced pancreatic parenchymal (a) and portal venous (c) phase images. Note that the pancreatic parenchyma is homogeneously and vividly enhancing during the pancreatic parenchymal phase (a), whereas a homogeneous decrease in the parenchymal enhancement degree is appreciable during the portal venous phase (b)
With CT, pancreatic adenocarcinoma typically appears hypoenhancing relative to the pancreatic parenchyma on early phase images and becomes less conspicuity compared to the adjacent pancreatic parenchyma on venous phase images (Fig. 7.6). This enhancement pattern is likely related to the desmoplasia and fibrosis, typically present within malignant pancreatic tumors [1, 8, 12, 15–17, 21–28]. Conversely, islet cell tumors, accounting for about 5 % of all pancreatic neoplasms, are generally small hyperenhancing masses that lack associated desmoplastic reaction [15–17, 21–28].
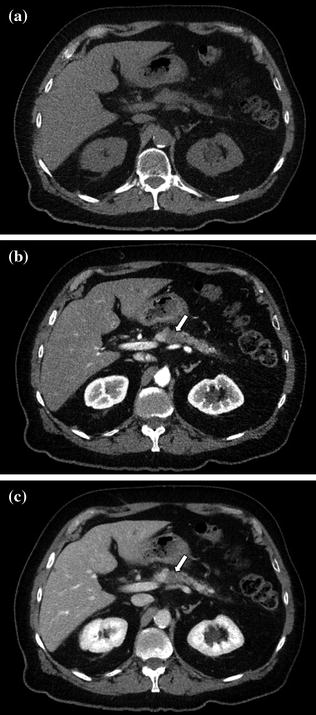

Fig. 7.6
Adenocarcinoma of the pancreatic body. The lesion is isodense to normal pancreatic parenchyma and therefore poorly identifiable on unenhanced image (a). By exploiting the maximum difference in attenuation between the poorly vascularized lesion and vividly enhancing pancreatic parenchyma, contrast-enhanced pancreatic parenchymal phase image (b) clearly shows a small lesion (arrow) in the pancreatic body. Although the tumor continues to be visible on the portal venous phase image (arrow) (c), the tumor conspicuity is lower compared to the pancreatic parenchymal phase image (b)
In terms of the diagnostic performance of multi-phasic CT for the detection of pancreatic tumors, initially there was no agreement on timing of the arterial phase acquisition [1, 8, 12, 15–17, 21–28]. Choi et al. [22, 30] reported that tumor detectability on arterial phase images obtained starting at 30 s after the initiation of contrast material was superior (95 %) to that on portal venous phase images obtained at 80 s (68 %). Conversely, Keogan [16] and Graf [17] reported that arterial phase images, when added to portal venous phase images, did not contribute to the improvement in the detection of pancreatic adenocarcinomas [16, 17, 22]. Meanwhile, Lu et al. first [18] introduced a new concept: pancreatic parenchymal phase imaging. This study suggested that the arterial phase images should be obtained beginning at 40 s after the initiation of contrast material during the so-called pancreatic parenchymal phase. This phase of enhancement consistently yields the highest lesion detection rate with optimal tumor conspicuity. The rationale for this observation is that CT images are acquired during peak pancreatic enhancement, when the maximum difference in attenuation is attained between poorly vascularized pancreatic tumors and vividly enhancing pancreatic parenchyma (Fig. 7.7) [18, 22]. Moreover, adequate mesenteric venous and arterial opacification for detection of vascular invasion is achieved during the pancreatic parenchymal phase of enhancement [29].
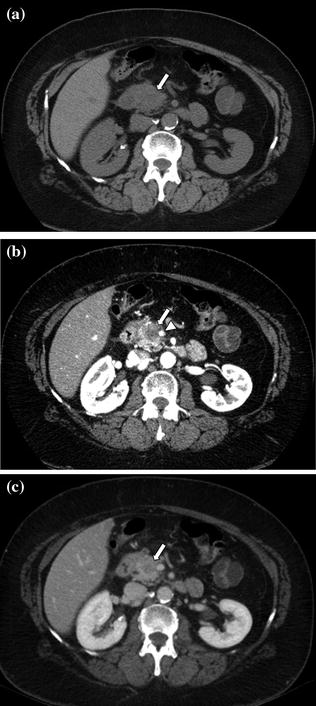

Fig. 7.7
Adenocarcinoma of the pancreatic head. An enlargement of the pancreatic head, associated with loss of lobular texture, convexity, and irregularities of contours (arrow), is visible on the unenhanced image (a). The pancreatic parenchymal phase image (b) clearly shows a hypodense mass (arrow) within the pancreatic head; the mass encases nearly 180° of the superior mesenteric artery (arrowhead). The mass (arrow) is ill-defined on the portal venous phase image (c)
Nowadays, most authors state that the initial phase of a contrast-enhanced biphasic pancreatic examination should be the pancreatic parenchymal phase (Fig. 7.8) [18, 29, 31]. Only a few authors advocate that the initial phase of the biphasic examination should be an earlier arterial phase [15–17]. The earlier arterial phase is reliable for depicting hyperenhancing lesions (i.e., most islet cell neuroendocrine tumors), which typically have early peak enhancement and rapid washout. However, since most islet cell tumors are also confidently identified during the pancreatic parenchymal phase, it is not necessary to have two different CT protocols [28, 29]. For this reason, there is general agreement that when a biphasic dynamic CT is performed, the earlier phase should be performed during the pancreatic parenchymal phase [14, 15].
Some centers use a small field of view to improve in-plane (x-axis and y-axis) spatial resolution and aid detection of small masses during the pancreatic parenchymal phase [8, 32]. This strategy has two important drawbacks: (1) exclusion of the outer portions of the liver, which must be evaluated for early-enhancing metastases particularly from an islet cell tumor, and (2) increased image noise due to the use of small voxels which may obscure small or subtle lesions [8].
The portal venous phase, during which there is maximum hepatic parenchymal enhancement, is usually performed as the later phase of a contrast-enhanced biphasic pancreas protocol. Imaging during the portal venous phase (70–90 s after the initiation of contrast material) provides optimal detection of hypoenhancing hepatic metastases from pancreatic adenocarcinoma as well as distant metastases and other ancillary findings such as lymph nodes, peritoneal implants, and malignant ascites (Figs. 7.9, 7.10, 7.11) [1, 4–8, 10–21]. The other advantage of imaging during the portal venous phase is the optimal delineation of the portal, splenic, and superior mesenteric veins [1, 8, 12–20, 25–28]. Thrombosis of the portal vein is a common complication of pancreatic adenocarcinoma, and determination of patency versus invasion has important implications for the resectability of pancreatic masses [8, 20]. Moreover, this study phase provides a second look at the pancreas, which may be helpful on occasion [1, 4–8, 10–21].
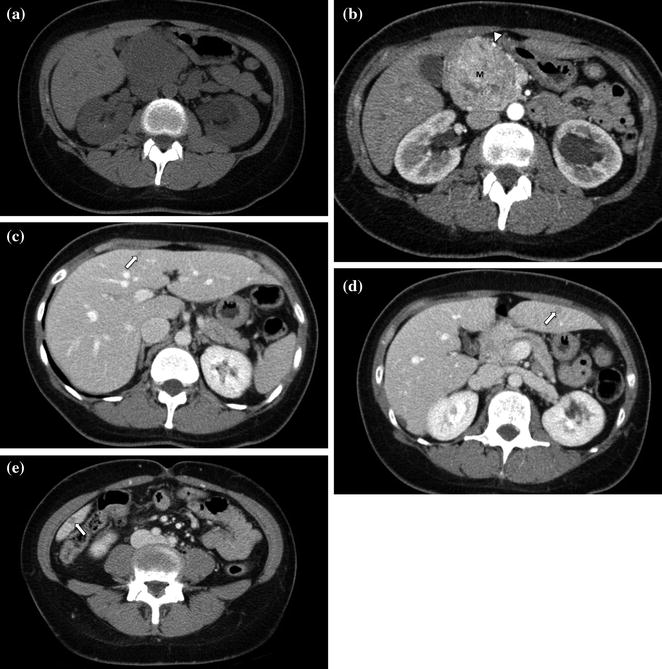
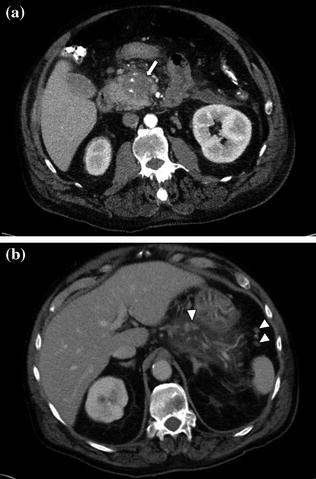
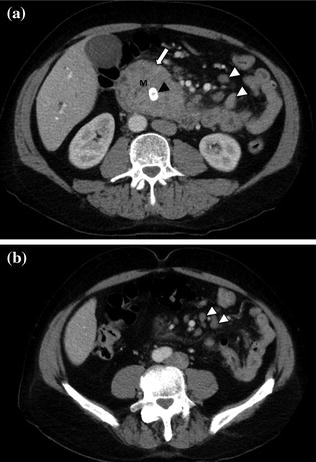

Fig. 7.9
Neuroendocrine tumor of the pancreatic head. An enlargement of the pancreatic head, associated with loss of lobular texture, convexity, and irregularities of contours (arrow), is visible on the unenhanced image (a). The mass (M) is vividly and heterogeneously enhancing on the pancreatic parenchymal phase image (b); note encasement of the gastroduodenal artery (arrowhead). Portal venous phase images (c–e) show multiple small hepatic metastases (arrows)

Fig. 7.10
Adenocarcinoma of the pancreatic head. Pancreatic parenchymal phase image (a) shows a hypoenhancing mass (arrow) with internal calcifications in the pancreatic head. Portal venous phase image (b) shows peripancreatic lymph nodes (arrowheads)

Fig. 7.11
a, b Adenocarcinoma of the pancreatic head. Portal venous phase image through the pancreatic head shows a large and moderately enhancing mass. Note the presence of a metallic biliary stent (black arrowhead) and several peripancreatic lymph nodes (white arrowheads)
After a pancreatic lesion is detected, the second goal for the radiologist interpreting the CT is preoperative tumor staging and likelihood of tumor resectability at surgery. In the absence of hepatic metastases or local tumor extension (i.e., invasion of adjacent organs), tumor resectability will depend on the presence of vascular involvement [33]. The superior mesenteric artery (SMA) is the most common artery involved in carcinoma of the pancreas, particularly for lesions arising from the pancreatic head (Fig. 7.12) [33, 34]. The celiac artery and its major branches are also frequently involved in pancreatic cancer, especially in larger tumors and in tumors of the pancreatic body and tail [33–35]. Arterial involvement significantly reduces the success of curative surgical resection [33]. Notably, limited tumor involvement of smaller arterial branches, such as the pancreaticoduodenal and gastroduodenal arteries, is generally not considered an absolute contraindication to surgery (Fig. 7.9) [33–35]. MDCT has an overall negative predictive value of 100 % for predicting vascular invasion, and a CT grading system of vascular involvement has been reported by Lu et al. [1, 36]. These authors prospectively graded vessel involvement using a 0- to 4-point scale based on the degree of circumferential contiguity of the tumor to the vessel (Grade 0: no contiguity, Grade 1: <25 % contiguity, Grade 2: 25–50 % contiguity, Grade 3: 50–75 % contiguity, Grade 4: >75 % contiguity) (Fig. 7.13). They found that when more than 50 % of the vessel’s circumference (grades 3 and 4) was in contact with a vessel, the likelihood of successful tumor resection was low [36], with a sensitivity and specificity for unresectability of 84 and 98 %, respectively [33–36].
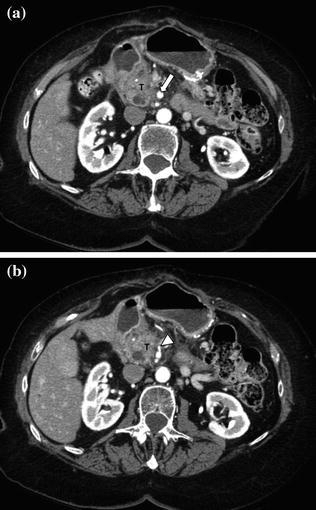
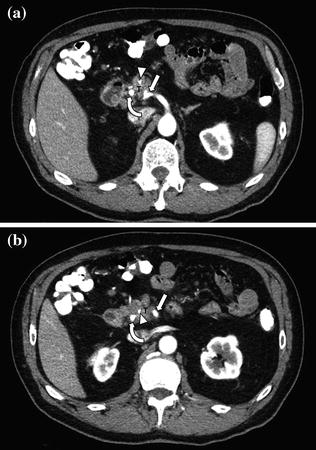

Fig. 7.12
Adenocarcinoma of the pancreatic head. Pancreatic parenchymal phase images (a, b) show a hypoenhancing mass in the head of the pancreas (T) with Grade 2 (<180°) tumor encasement of the right side of the superior mesenteric artery, as well as on the proximal branches (arrowhead)

Fig. 7.13
Adenocarcinoma of the pancreatic head. Pancreatic parenchymal phase images (a, b) show Grade 2 (90–180°) contiguity of the tumor (T) with the proximal superior mesenteric artery (arrow). Note the presence of common bile duct (curved arrow) and main pancreatic duct stents (arrowhead)
The assessment of venous structures such as the portal vein, superior mesenteric vein, and splenic vein also plays an important role in determining the feasibility of surgery in patients with pancreatic cancer. The CT grading system described by Lu et al. [36] can be also applied to veins [33, 36, 37]. Unequivocal invasion of the venous wall by tumor, rather than simple contiguity, is generally necessary to assess resectability. Tumor involvement of larger veins, such as the portal vein and/or superior mesenteric vein, is generally regarded as a contraindication for surgery. Isolated involvement of the proximal (upstream) portal vein, however, will not necessarily preclude pancreatic surgery as some institutions will follow tumor resection with a venous graft reconstruction [37].
Lymphatic channels within the pancreas drain into lymph nodes around the celiac axis and the superior mesenteric vein. As these lymphatic vessels become engorged with tumor cells, they produce a perivascular cuff of soft tissue that represents extrapancreatic disease [20]. On occasion, this perivascular cuff may be the only radiologic sign of a pancreatic carcinoma [20]. The assessment of lymph nodes in pancreatic tumors deserves a special mention. CT is inaccurate for the detection of regional lymph node metastases [1, 40–42]. The CT criteria for nodal involvement, based on size, are less reliable as large lymph nodes may be hyperplastic and normal-sized lymph nodes may have microscopic metastases. The low detection rate of regional lymph node metastases has limited clinical implications, as peripancreatic lymph nodes are routinely resected at surgery [1, 40–42]. Moreover, regional lymph node metastasis is not a contraindication for surgical resection if there is no evidence of vascular invasion or distant metastases [1, 40–43].
Three-dimensional volume display of CT angiographic datasets also provides a comprehensive display of the key arterial and venous anatomy needed to properly determine resectability of pancreatic cancer [33]. Curved planar reformatting (CPR), maximum intensity projection (MIP), or volume rendering can be particularly advantageous for evaluating the peripancreatic vasculature (Fig. 7.14) [33–35, 38].
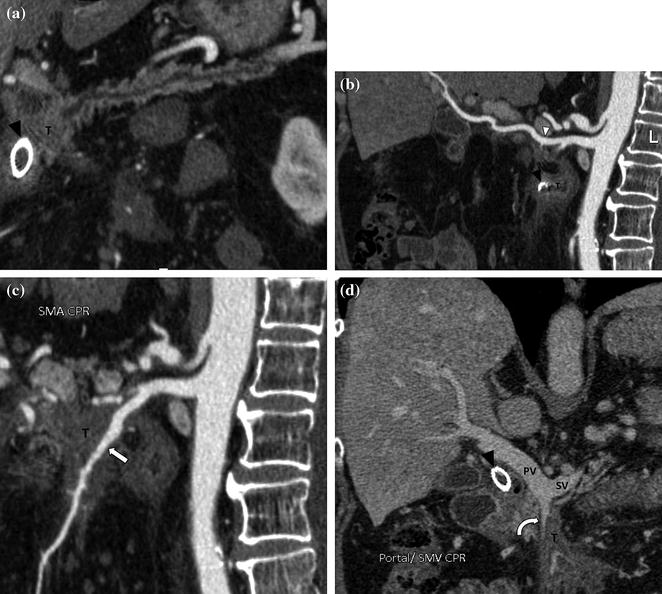

Fig. 7.14
Benefits of a CPR image in advanced adenocarcinoma of the pancreatic head. Pancreatic parenchymal phase CPR image through the pancreas (a) shows the presence of a tumor (T) in the pancreatic head with upstream main pancreatic duct dilatation. Note the presence of a metallic stent in the common bile duct (black arrowhead). Pancreatic parenchymal phase CPR image through the course of the right hepatic artery (b) depicts perivascular tumor extension (arrowhead). Note that in this patient, the right hepatic artery arises from the superior mesenteric artery, whereas the left hepatic artery arises from the celiac trunk. Pancreatic parenchymal phase CPR image (c) through the course of the superior mesenteric artery (arrow) shows Grade 4 (>270°) contiguity of the tumor (T). Portal venous phase CPR image (d) through the portal confluence also depicts >270° contiguity of the tumor with the superior mesenteric vein (curved arrow). The portal vein (PV) and the splenic vein (SV) are not involved
Finally, the staging of pancreatic lesions should always provide information about hepatic metastases, local tumor extension to the adjacent viscera, such as stomach and colon, and peritoneal deposits. All these features preclude surgical resection [38, 39].
Despite the developments of new-generation MDCT scanners, approximately 11 % of pancreatic ductal adenocarcinomas still remain undetected by CT because of the lack of a visible attenuation difference between the lesion and the adjacent pancreatic parenchyma. These lesions may be detectable only by identification of a contour abnormality or segmental dilation of the pancreatic duct [9].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree