Dheeraj Rajan • Ricardo Yamada
In 2006, the last update of the Kidney Disease Outcomes Quality Initiative along with the Fistula First program reinforced the concept of arteriovenous fistula (AVF) as the preferred hemodialysis (HD) access, given its longer durability, less risk of infection, and less overall cost compared with arteriovenous grafts (AVGs).1,2 Unfortunately, AVF primary failure can reach up to 53% according to one series.3 Therefore, multiple strategies have been implanted to improve outcome, including better patient selection, refined surgical techniques, and access surveillance. Recently, endovascular techniques have been successfully used to improve AVF development, including collateral veins embolization. In addition, primary failure is not the only obstacle encountered among HD accesses, and percutaneous embolization has also been applied to solve other access-related problems such as arterial “steal syndrome” and traumatic fistula formation adjacent to main AVG.
NONMATURED FISTULA
Anatomic Considerations
The ideal AVF is the one that can be easily cannulated, providing sufficient blood flow with satisfactory long-term patency. Objective parameters have been established to evaluate those characteristics, and they include minimal flow of 600 mL per minute, diameter bigger than 6 mm, and location approximately 6 mm from the skin surface.4 To achieve these goals, quality of the vessels being used is a key factor. The minimum diameter before AVF creation for arteries should be 2.0 mm and for veins 2.5 mm, as shown by Wong et al.5 who demonstrated that fistulas created with vessels smaller than 1.6 mm were associated with early failure. Also, too calcified arteries and/or preexisting focal venous/arterial stenoses can prevent fistula maturation.
The most common and preferred AVF is the radiocephalic fistula, which is created just above the wrist. The cephalic vein is cut and the portion going up to the forearm is anastomosed to the side of the radial artery in an end-to-side anastomosis. The distal end directed to the hand is then ligated. The second most used AVF is the brachiocephalic fistula, created just above the elbow. Again, this is an end-to-side anastomosis, in which the proximal end of the cephalic vein is anastomosed to the side of the brachial artery. The distal cephalic vein is ligated. Finally, the least common AVF is the brachiobasilic fistula, in which the end of the basilic vein is anastomosed to the side of the brachial artery. Because the basilic vein has a deep course, it needs to be mobilized to the subcutaneous tissue where it can be easily accessed.
Pathophysiology
Basically, fistula creation bypasses a high-resistance vascular bed, diverting blood flow toward a low-resistance venous circuit, which is now receiving “arterialized” inflow. In response to this new environment, progressive arterial and vein dilation are expected over time to accommodate this high blood volume in this new low-resistance system. Initially, vein dilation results from the increased blood pressure within it. In addition, a second component plays an important role and that is called beneficial vascular remodeling.6,7
In this process, the increased blood flow induces an increased longitudinal shear stress against the vein wall, and this promotes endothelial cell quiescence and secretion of anti-inflammatory and anticoagulant agents. This will, ultimately, result in vein dilation and reduction in neointimal hyperplasia. On the other hand, reduction in both blood flow and longitudinal shear stress leads to endothelial cell activation and release of inflammatory and procoagulant factors, which will result in neointimal hyperplasia and vasoconstriction, an opposite process called negative vascular remodeling. Another important part of this process is the degree of medial hypertrophy, which is determined mainly by the transmural pressure. It has been demonstrated that increased intraluminal pressure activates smooth muscle cells, cytokine expression, and production of extracellular matrix components.8 The final result is thickening of the muscular layer of the vessel wall. Therefore, fistula final lumen diameter is determined by the combination of three components: vein dilation, neointimal hyperplasia, and medial hypertrophy. Most of the times, lumen loss due to intimal hyperplasia and medial hypertrophy is compensated by vein dilation, and the final diameter is large enough to promote the necessary blood flow for an efficient dialysis.
Blood flow through the fistula is the major determinant of beneficial vascular remodeling and, in consequence, fistula maturation. Some anatomic problems can prevent adequate flow within the fistula, halting fistula development, and the most common are stenotic lesions and presence of accessory veins.9 According to Beathard et al.,9 outflow venous stenosis is found in 78% of the patients with a failed-to-mature fistula, and presence of accessory veins is found in 46% of patients. In these scenarios, percutaneous balloon angioplasty has been proven to be a useful tool in facilitating fistula maturation and, more recently, accessory vein embolization has become a valuable option.
CLINICAL APPLICATIONS
Nonmatured Arteriovenous Fistula Embolization
It has been confirmed that presence of accessory veins is the second most anatomic factor that prevents fistula maturation.9,10 In the setting of an AVF, accessory veins divert flow from the main venous channel, which in turn reduces the resistance and blood flow within the venous segment above the branch(es). Flow reduction leads to decreased longitudinal shear stress, which triggers negative vascular remodeling, causing exacerbated neointimal hyperplasia and vasoconstriction. Ultimately, AVF maturation may be compromised. According to Turmel-Rodrigues et al.,11 filling of an accessory vein would not be a cause of poor fistula maturation but only a consequence of an underlying outflow venous stenosis, therefore vein obliteration would not be necessary as long as the stenotic lesion is fixed. Nonetheless, Beathard et al.10 in their series of 100 patients described 12 failed fistulas in which isolated accessory veins without outflow stenoses were the only anatomic culprit factors. All of them were obliterated with 100% success rate. Accordingly, other series have shown that accessory vein obliteration was effective in promoting fistula maturation.12,13
Thus, obliteration of those accessory veins has been applied when AVF fails to mature despite venous stenosis correction or when those accessory channels are the only anatomic abnormality. Initially, vein ligation was the only option. For that, after fistulography, accessory vein location is identified and marked on the skin surface. A small incision is made and after fluoroscopic vein visualization, ligation is performed with 4-0 silk and the skin incision is closed with 4-0 sutures. A less invasive technique has been described without skin incision using two 2-0 polypropylene sutures and inserting the needles into the skin adjacent to the vein and advancing them under the accessory vein until they reach the skin on the opposite side. The notches are then set on the skin surface and ligation is confirmed with a fistulogram. With these techniques, successful fistula maturation has been described as high as 100% with the first technique9 and 88 % with the last one.12
Vein ligation is an excellent option for superficial veins as they can be easily reached with a small surgical incision or even transcutaneously. For deeper veins, ligation is not well suited as damage to muscles, nerves, or tendons can occur. For that location, embolization is a better option because these veins can be selectively catheterized and then occluded. On the other hand, coil embolization of very superficial veins can lead to skin irritation/erosion given the close proximity between the embolic agent and cutaneous tissue (Fig. 28.1).

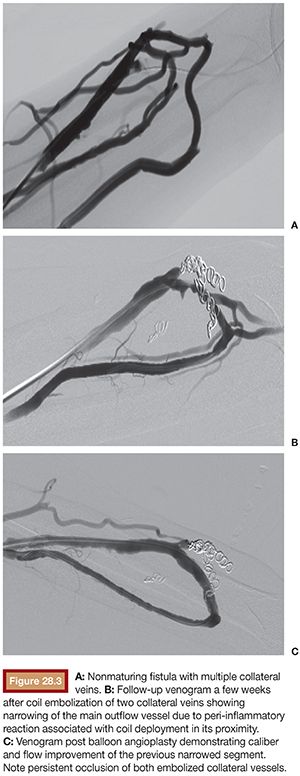
Embolization has been found to be a valid option by Nikam et al.,13 who treated dysfunctional fistulas with coil embolization alone or in combination with angioplasty when necessary, achieving fistula maturation in 10 out of 14 patients. The technique for fistula embolization has been previously described, with the use of pushable fibered coils as the preferred embolic device.14 To prevent distal coil migration, at least 2-mm upsizing should be applied and, ideally, compact packing should be obtained to occlude flow efficiently (Fig. 28.2). Another important technical point is to maintain a safe distance from the point of embolization and the main outflow vein. There should be at least 5-mm distance to avoid outflow vein stenosis due to postembolization inflammatory reaction and also to prevent misplacement of the embolic agent within the main outflow vein (Fig. 28.3).

Other options of embolic devices, taking into consideration a more controlled deployment, are detachable coils and the Amplatzer Vascular Plug (St. Jude Medical, Inc., St. Paul, Minnesota). Powell et al.15 reported using the Amplatzer Vascular Plug to occlude a tributary vein that was diverting flow from the cephalic vein and preventing fistula maturation. This device is made of a self-expandable nitinol mesh that expands within the vessel, achieving the necessary wall apposition. This helps secure the device in place, avoiding distal embolization. For that, the manufacturer advises 30% to 50% upsizing to achieve ideal wall apposition and safe deployment. In addition, the delivery mechanism permits deployment, retrieval/reposition, and redeployment of the plug, if necessary. The second generation of the device, the Amplatzer Vascular Plug II, is believed to promote faster vessel occlusion compared with the first generation15 due to increased surface area and thrombogenicity. The device size varies from 3 to 22 mm, which requires up to a 7-Fr sheath or 9-Fr guiding catheter. The latest generation, the Amplatzer Vascular Plug 4, has a low-profile system, allowing delivery of up to a 8-mm plug through a 5-Fr diagnostic catheter.
Embolization for Steal Syndrome
Steal syndrome occurs when the AVF prevents adequate blood supply to the hand. It is a rare complication and its incidence ranges from 1.7% to 8%, depending on multiple factors, with fistula location being a major factor.16 AVFs at the level of the elbow have higher incidence of steal syndrome compared with the ones located on the wrist. Symptoms and signs include numbing, tingling, pain, and weakness; decreased temperature; and diminished pulses. In severe cases, ulceration can occur. A high index of suspicion should be maintained as those signs and symptoms may be nonspecific and misinterpreted as diabetic neuropathy.
Initially after fistula creation, there is reduction of the distal tissue perfusion, which in turn induces collateral circulation development and peripheral vasodilation. These compensatory mechanisms in combination with a competent ulnar artery and palmar arch permit adequate tissue perfusion in most cases. Unfortunately, in a minority of patients, the hemodynamic changes after AVF creation are not adequately compensated by those mechanisms, leading to insufficient distal blood perfusion to meet the metabolic requirements.
This new hemodynamic environment is part of the complex pathophysiology of steal syndrome, in which the major determinant is the difference between fistula’s resistance and the resistance of the hand’s capillary circulation. Because fistula resistance is lower than the resistance of the distal capillary bed, most or even all blood from the donor artery will be diverted into the AVF, but that should not be an issue, as the ulnar artery and the palmar arch can guarantee adequate hand perfusion (Figs. 28.4 and 28.5A).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








