CHAPTER 12
Ebstein Anomaly
Definition
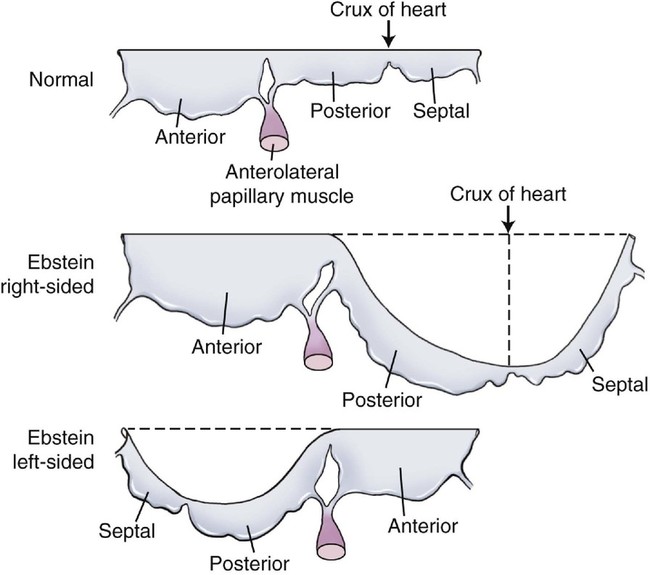
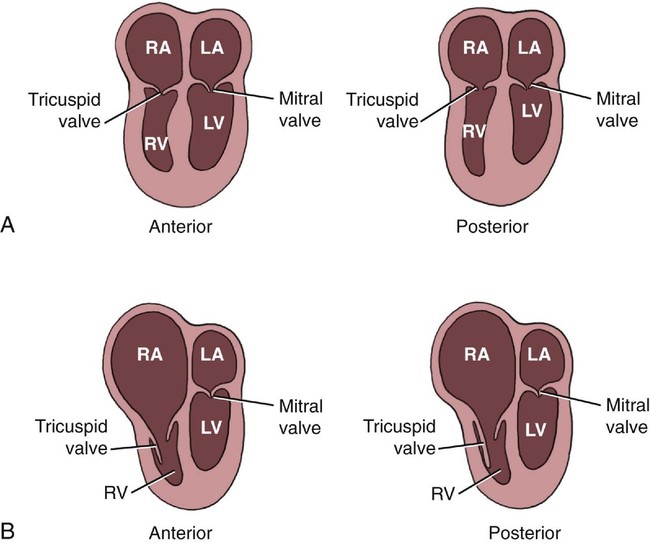
Ebstein anomaly is characterized by apical displacement of the septal, and usually the posterior, leaflet of the tricuspid valve into the right ventricle.1 The degree of this displacement is inconsistent.2 In addition, the septal and posterior leaflets of the tricuspid valve are adherent to the ventricular wall to a variable degree so that the relatively small, mobile portions of the leaflets may reside deep within the right ventricle. If there is substantial adherence of the leaflets to the right ventricular wall, only a small part of the valve cusps will be free to move. This contributes to tricuspid insufficiency.
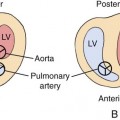
The maximal amount of valve displacement occurs at the crux cordis, the commissure between the septal and posterior leaflets (Fig. 12–1). This inferior displacement of the tricuspid valve results in “atrialization” of the inflow portion of the right ventricle so that it behaves functionally like part of the right atrium. This atrialized portion of the right ventricle may have little or no residual myocardium and may closely resemble the right atrium associated with Uhl malformation. Frequently the right atrium is severely dilated. The nonatrialized apical portion of the right ventricle generally demonstrates a normal wall thickness but may be dilated.
In addition to the displacement, some degree of tricuspid dysplasia is virtually always present in the neonatal expression of Ebstein anomaly. This dysplasia may range from mild to severe.2,3
Last, the distal attachments of the anterior and septal leaflets of the tricuspid valve may be abnormal with thickened chordae and abnormal linear attachments between the valve cusps and the trabecula of the right ventricle, tethering the leaflet to the ventricular wall.1 When there are numerous linear attachments, functional tricuspid stenosis can result. In its most severe form, this can mimic tricuspid atresia and has been termed the imperforate type of Ebstein anomaly.4–6 In most cases of Ebstein anomaly, however, the displacement, dysplasia, and abnormal distal attachments of the tricuspid valve result in tricuspid insufficiency, which causes further enlargement of the right atrium and the right ventricle.
Ebstein anomaly is one of the few congenital heart defects to cause significant cardiac dysfunction in utero, frequently with resultant severe cardiomegaly, hydrops fetalis, and tachyarrhythmias.7
Embryology
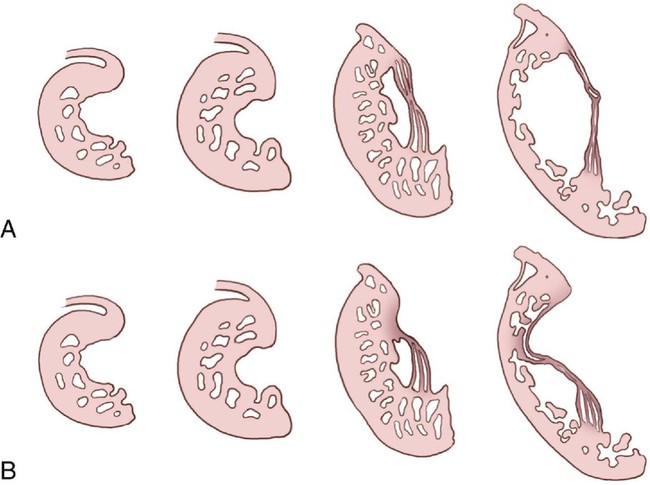
The tricuspid valve leaflets and chordae tendineae are believed to be formed by undermining of the inner layers of the right ventricular inlet, a process called delamination (Fig. 12–2).8 The valve leaflets and chordae tendineae are initially muscular structures but later develop fibrous components. The posterior and septal leaflets are formed at 12 to 16 weeks’ gestation. In Ebstein anomaly, the delamination process is faulty, resulting in the abnormal apical insertion of the septal and posterior tricuspid valve leaflets at the junction of the inlet and trabecular portions of the right ventricle. The anterior leaflet is formed much earlier in embryological development and also has a muscular origin. It may contain various amounts of muscle and can even consist of a complete muscular diaphragm between the inlet and apical portions of the right ventricle. Zuberbuhler et al4 suggested that the anterosuperior leaflet arises from the trabecula septomarginalis.
Occurrence Rate
Ebstein anomaly is one of the less common congenital heart defects, accounting for 3% to 7% of cases of congenital heart disease in the fetal population.9,10 It occurs in approximately 1 in 20,000 live births11 and makes up 0.5% of congenital cardiac disease cases.12 Males and females are affected equally.13 Although the anomaly generally appears sporadically, there have been reports of familial Ebstein anomaly.14–18 The risk of occurrence when one sibling is affected is approximately 1%.19
Ebstein anomaly has been associated with maternal ingestion of lithium carbonate, a first-line drug used to treat manic-depressive psychosis, suggesting that lithium is a specific teratogen.11,20–24 Most data supporting this view came from the Danish Registry, in which more than 200 “lithium babies” (normal or congenitally abnormal babies born to mothers who ingested lithium during the first trimester of pregnancy) were registered. In this group, there was a 10% incidence of congenital heart disease compared with the 0.01% risk in the general population. Importantly, the Danish Registry recorded eight cases of Ebstein anomaly. Because Ebstein anomaly occurs sporadically in 1 in 20,000 live births, this suggested that maternal lithium ingestion increased the risk of Ebstein anomaly 500-fold over the incidence in the general population.
The first major study that attempted to avoid this bias reported cardiac malformations in 7% of lithium-exposed fetuses but failed to link lithium with Ebstein anomaly.24
A case-control study on the association between first-trimester lithium exposure and Ebstein anomaly suggests that, if lithium increases the risk of Ebstein anomaly, the increased risk is not more than 28-fold—substantially less than the 500-fold increase estimated by the data from the lithium registries.25 More recent retrospective, prospective, and meta-analysis studies failed to show a clear association between Ebstein anomaly and lithium.26
Sonographic Criteria
The diagnosis of Ebstein anomaly is readily made in utero.7,27,28 Oberhoffer et al28 retrospectively reviewed 19 postmortem cases of Ebstein malformation or tricuspid valvular dysplasia in fetuses who underwent echocardiography between 16 and 37 weeks’ gestation. Death occurred between 20 and 40 weeks’ gestation, and the time between fetal echocardiography and fetal death ranged from 1 to 12 weeks. This study clearly demonstrates that fetal echocardiography can not only detect but also can reliably differentiate various types of tricuspid valvular disease in utero.
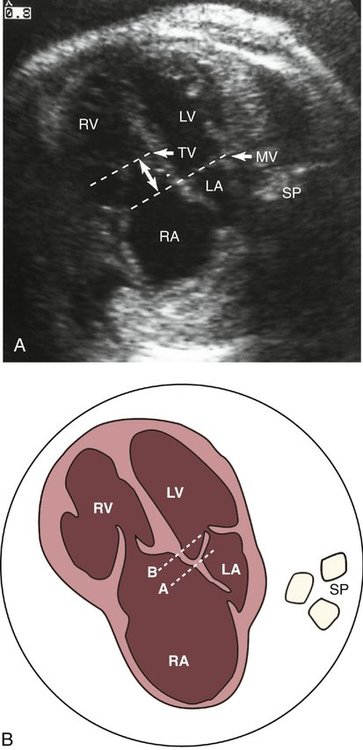
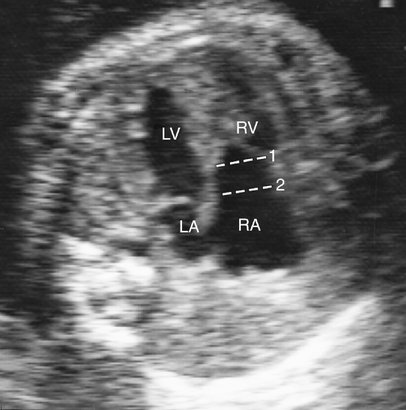
Although an enlarged right atrium may be the first indication that congenital heart disease is present, apical displacement of the tricuspid septal leaflet is the most reliable sign of Ebstein anomaly. This displacement can be recognized by comparing the level of the insertion of the septal leaflet of the tricuspid valve with that of the mitral valve and determining the amount of offset between the two (Fig. 12–3). Several methods have been described to quantify the degree of tricuspid valve displacement. Ports et al29 compared the mitral valve-to-apex and the tricuspid valve-to-apex distances (Fig. 12–4). This method becomes invalid when the size of either ventricle is abnormal. This group also measured the distance between the displaced tricuspid valve and the atrioventricular groove (Fig. 12–5). Because it is sometimes difficult to localize the atrioventricular groove reliably by ultrasonography, this method can be inaccurate.
Kambe et al30 described the simplest and most reliable method of determining septal leaflet offset. They simply measured the distance between the septal leaflet of the tricuspid valve and the anterior leaflet of the mitral valve (Fig. 12–6). This can be accomplished in the subcostal or apical four-chamber view.
Because the insertion of the septal leaflet of the tricuspid valve is slightly more apical than that of the anterior mitral valve leaflet in the normal heart, the diagnosis of mild forms of Ebstein anomaly may be difficult. It is important to identify a cutoff value between normal and abnormal offsets. Gussenhover et al31 determined the minimal and maximal offset of the septal leaflets of the mitral and tricuspid valves anatomically and with echocardiography in fetuses, infants, children, and adults with and without Ebstein anomaly. In the first trimester of pregnancy, it was impossible to detect any offset between the tricuspid and mitral valves in normal fetuses. Thereafter, the distance gradually increased in the normal heart; however, accurate measurements were not established for normal fetuses in the second and third trimesters of pregnancy. In normal infants, the offset was less than 8 mm, in normal children it was less than 15 mm, and in normal adults it was less than 20 mm.31
Oberhoffer et al28 studied a group of fetuses with tricuspid valvular disease between 16 and 37 weeks’ gestation. In two fetuses with pathologically proven Ebstein anomaly, the offset was only 5 mm. Displacement varied between 4 and 20 mm in 10 cases of fetal and neonatal Ebstein anomaly in the series of Lang et al.32
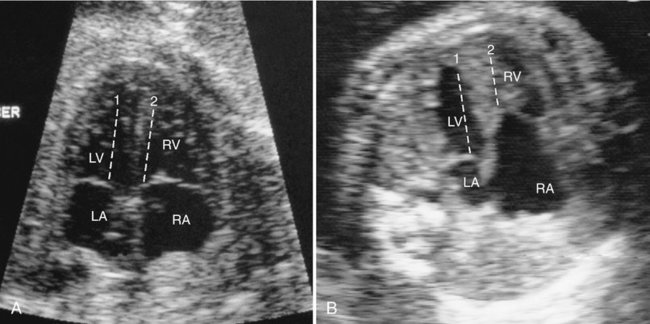
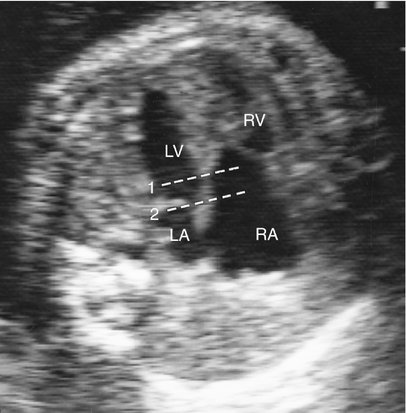
In the normal heart, the offset between the septal insertions of the mitral and tricuspid valves is maximized, with anterior angulation best visualized in the subcostal four-chamber view. In the heart with Ebstein anomaly, this relationship is reversed so that the offset of the atrioventricular valves is accentuated with posterior angulation of the subcostal four-chamber view.31 This observation is important and can be particularly helpful in the diagnosis of milder forms of Ebstein anomaly (Fig. 12–7).
In some instances, a four-chamber view may appear normal in the setting of milder forms of Ebstein anomaly if the tricuspid valve orifice is mistaken for the valve itself (Fig. 12–8, A
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree