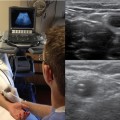
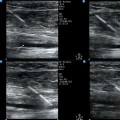
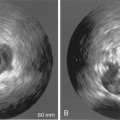
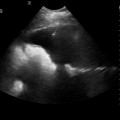
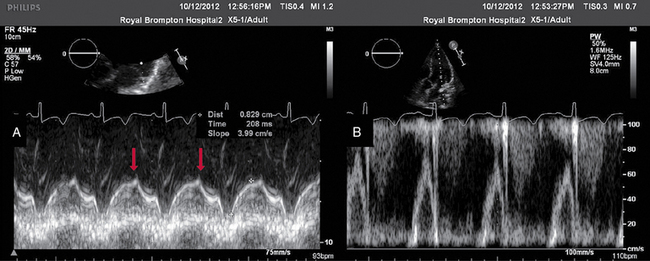
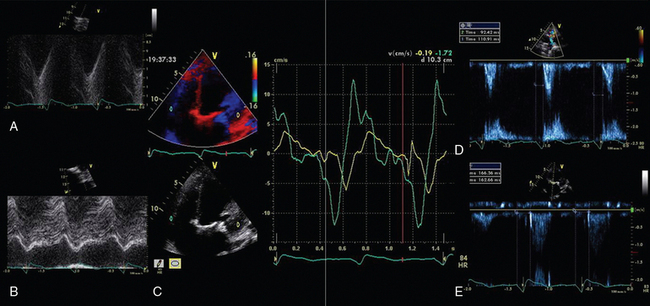
28 Echocardiography is one of the most powerful diagnostic and monitoring tools available to the modern intensivist. Although its potential was first recognized more than 20 years ago,1 only recently has it become a mainstream imaging application in the intensive care unit (ICU). However, the widespread application of advanced echocardiographic techniques in the ICU remains disappointingly limited to isolated experts across the world, and the huge potential for collaborative research between cardiologists and intensivists in this field remains largely unrecognized. The reasons for this are largely historical; cardiology and intensive care have developed in parallel, with little overlap between the two specialties. With the paradigm shift in cardiologic interventions (percutaneous coronary, valve, and electrophysiologic procedures), there has been an understandable change in focus away from the more traditional specialties based upon cardiac physiology toward the newer interventional subspecialties. The development of complex and sophisticated imaging techniques (cardiac magnetic resonance and computed tomography) has presented further competition to echocardiography as a subspecialty within cardiology. Controversies regarding practice, training, accreditation, and “ownership” have until recently increased the potential conflict between cardiologists and intensivists regarding the use of echocardiography in the ICU (Table 28-1). Nonetheless, increasing availability of ultrasound machines, together with concerns regarding the safety of the pulmonary artery catheter, have resulted in intensivists adopting echocardiography as part of their repertoire of diagnostic and monitoring techniques. This chapter illustrates some neglected physiologic echocardiographic techniques as well as some of the potential areas where newer techniques might prove to be of use in the ICU. TABLE 28-1 Basic and Advanced Echocardiographic Techniques in the ICU The mechanism of LV function in systole and diastole is complex, with differing orientation of fibers at different muscle layers, and comprises minor and long-axis contraction, rotational contraction, as well as differential basal and apical rotational vectors. Of the older techniques, assessment of LV contractility generally either depends upon linear measures of changes in LV internal dimensions (fractional shortening) or differences between systolic and diastolic areas/volumes in the minor axis (ejection fraction), rather than thickening of the myocardium per se. Normal values of fractional shortening and ejection fraction are not known for the critically ill and remain highly variable depending upon ICU interventions in addition to the inherent contractility of the myocardium. The true value of other, less widely used techniques (long-axis function and total isovolumic time) has not yet been realized in the ICU setting, and that of emerging techniques (strain and strain rate) remains to be evaluated. The subendocardial fibers of the left ventricle run longitudinally, resulting in the characteristic movement of the mitral annulus toward the apex of the heart in systole, followed by retraction in diastole. Regional myocardial ischemia (induced by positive inotropic agents) may result in changes in regional wall motion scores associated with a fall in cardiac output at peak stress.2 Regional dyssynchrony may also develop, demonstrated by the appearance/worsening of postejection shortening, prolongation of the total isovolumic time (t-IVT), and associated broadening of the QRS duration. This response is in contrast to the normal shortening of the QRS duration and t-IVT in response to inotropic agents. Thus there is potential to diagnose subclinical myocardial ischemia/type I/II myocardial infarction by using this simple technique (Figure 28-1). Further, because t-IVT is a major determinant of maximal cardiac output during pharmacologic stress, it may be possible to titrate, on an individual patient basis, the response to positive inotropic agents, to minimize electromechanical dyssynchrony and avoid limiting cardiac output (Figure 28-2). Figure 28-1 TTE in a young patient with type II myocardial infarction. A, MAPSE of the lateral wall, with postejection shortening (red arrow). B, PW Doppler of the mitral valve showing abnormal filling with summation (resulting from tachycardia) and a dominant A wave. MAPSE, Mitral annular plane systolic excursion; PW, pulsed wave; TTE, transthoracic echocardiogram. Figure 28-2 Interventricular dyssynchrony demonstrated by M-mode, TDI, and PW Doppler; A and B, M-mode traces of the right (TAPSE) and left (MAPSE) ventricle, depicting prolonged and late movement of the LV when compared with the RV free wall, as well as reduced excursion. C, PW TDI of the right (green) and left (yellow) ventricle taken from the illustrated regions (miniature images to the left; namely the LV and the RV free wall, respectively). LV TDI velocities are reduced and delayed. D, PW Doppler taken from the pulmonary valve showing a preejection period of 98 msec. E, PW Doppler from the aortic valve, showing a preejection period of 164 msec. Thus echo demonstrates a significant delay in LV contraction compared with the RV, resulting in a delay in ejection. LV, Left ventricle; MAPSE, mitral annular plane systolic excursion; PW, pulsed wave; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue Doppler imaging. Factors increasing this measure of global LV electromechanical dyssynchrony include ischemia, conduction system disturbances, and loading conditions. Although there is not extensive literature regarding resynchronization therapy (atrioventricular [AV] and/or ventriculoventricular [VV]) in the ICU, the effects of dyssynchrony are well-recognized (including reduced LV contractility [dP/dT], pulse pressure, ejection fraction and cardiac output, diastolic filling time, and increased duration of mitral regurgitation and t-IVT). Even simple manipulations of heart rate and AV delay can result in significant improvement in cardiac filling (Figure 28-3). Global LV electromechanical dyssynchrony measured by t-IVT is common (22% patients in cardiothoracic ICUs) and is associated with increased mortality (7.5% vs. 25%, P
Echocardiography: Beyond the basics
(CONSULTANT-LEVEL EXAMINATION)
Overview
Technique
Routine ICU Use Recommended?
Comments
Focused basic echocardiography
Yes
Easy, binary decision-making to exclude/diagnose treatable causes of periarrest and arrest states
Ejection fraction
No
Normal values unknown in ICU
Too many dynamic variables
Does not measure contractility
Long-axis function
Yes
Easy to acquire (even with suboptimal views)
Reproducible
Sensitive to ischemia
Physiologic Doppler
Yes
Assessment of standard echodynamics
Evaluation of t-IVT
Strain/strain rate
No
Research tool
Not validated in critically ill adults
Image resolution an issue
3D/4D
Yes: for specific indications
Delineation of paraprosthetic regurgitation
Assessment of mechanism of MR (percutaneous repair)
Right ventricular function/dyssynchrony (research)
Left ventricular (LV) function
Long-axis function
Total isovolumic time (t-IVT)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine