Effects of Radiation on the Embryo and Fetus
▪ HISTORICAL PERSPECTIVE
In the early years of the 20th century, case reports began to appear in the medical literature that described mental retardation in children with small head size, as well as other gross malformations, born to mothers who had received pelvic radiotherapy before realizing that they were pregnant. As early as 1929, Goldstein and Murphy reviewed 38 such cases. Interestingly enough, they concluded that large doses were needed to produce such effects and did not consider diagnostic pelvic irradiation of the mother to be a hazard.
We now have a great deal of information concerning the effects of radiation on the developing embryo and fetus from both animal experiments and the human experience.
▪ OVERVIEW OF RADIATION EFFECTS ON THE EMBRYO AND FETUS
Among the somatic effects of radiation other than cancer, developmental effects on the unborn child are of greatest concern. The classic effects are listed as follows:
Lethal effects are induced by radiation before or immediately after implantation of the embryo into the uterine wall or are induced after increasingly higher doses during all stages of intrauterine development, to be expressed either before birth (prenatal death) or at about the time of birth (neonatal death).
Malformations are characteristic of the period of major organogenesis in which the main body structures are formed, and especially of the most active phase of cell multiplication in the relevant structures.
Growth disturbances and growth retardation, without malformations are induced at all stages of development but particularly in the latter part of pregnancy.
The principal factors of importance are the dose and the stage of gestation at which it is delivered. Dose rate is also of significance because many pathologic effects on the embryo are reduced significantly by reducing the dose rate.
It should be recognized that congenital anomalies arise in all animal species, even in the absence of any radiation beyond that received from natural sources. The incidence depends largely on the time at which the anomalies are scored. In humans, the average incidence of malformed infants at birth is about 6%. Some malformations disappear after birth, but more become evident later that are not scored at birth. The global incidence roughly doubles to 12% if grown children rather than infants are examined. Any assessment of the effectiveness of radiation in inducing damage in utero must be viewed against this natural level of inborn defects and its variable expression.
▪ DATA FROM MICE AND RATS
Most experimental data on the effect of radiation on the developing embryo or fetus have been obtained with the mouse or rat, animals that reproduce in quantity with relatively short gestation periods. Russell and Russell divided the total developmental period in utero into three stages: (1) preimplantation, which extends from fertilization to the time at which the embryo attaches to the wall of the uterus; (2) organogenesis, the period during which the major organs are developed; and (3) the fetal period, during which growth of the structures already formed takes place. There is a very large variability in the relative duration of these periods among animal species, as well as in the total duration of intrauterine life. In addition, at any given stage of development, the state of differentiation or maturation of any one structure, with respect to all the others, varies considerably in different species.
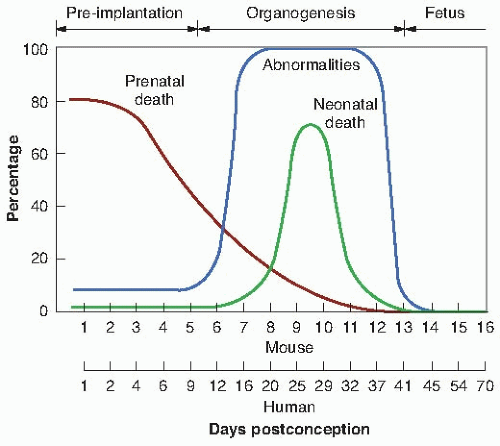
In the mouse, preimplantation corresponds to days 0 through 5; organogenesis, days 5 through 13; and the fetal period, day 13 through full term, which is about 20 days. The effect of about 2 Gy of x-rays delivered at various times after conception is illustrated in Figure 12.1. The lower scale contains Rugh’s estimates of the equivalent ages for human embryos, based solely on comparable stages of organ development. It is a nonlinear match because preimplantation, organogenesis, and the fetal period in the mouse are about equal in length, whereas the fetal period in the human is proportionately much longer.
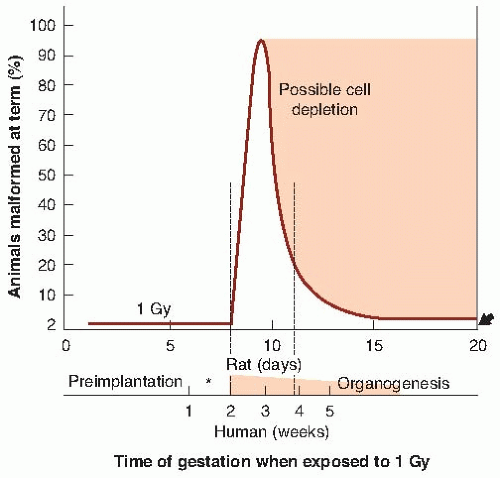
Figure 12.2 is taken from the work of Brent and Ghorson, who performed an extensive series of experiments with rats. It shows the various periods during gestation in which the principal effects of radiation are most evident. The horizontal scale refers to the times of the major events during gestation for the rat and gives an estimate of the comparable stages for the human. The following discussion of the principal effects of radiation delivered during preimplantation, organogenesis, and the fetal stages represents a consensus view, combining conclusions from various experiments with either rats or mice.
Preimplantation
Preimplantation is the most sensitive stage to the lethal effects of radiation. The high incidence of prenatal death may be expressed in a decrease in litter size. Growth retardation is not observed after irradiation at this stage; if the embryo survives, it grows normally in utero and afterward. Few, if any, abnormalities are produced by irradiation at this stage. The International Commission on Radiological Protection (ICRP) Publication 103 confirms embryonic susceptibility to the lethal effects of irradiation in the preimplantation period of embryonic development, but suggests that at doses less than 100 milligrays (mGy), such lethal effects will be very infrequent in the human.
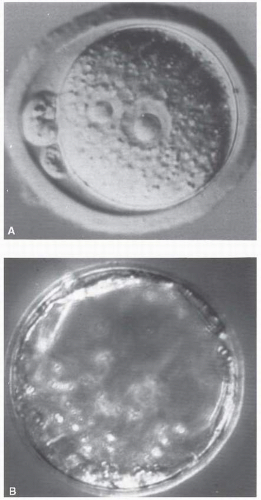
Thus, the irradiated preimplanted embryo that survives grows normally, that is, there is an “all-or-nothing” effect of radiation because if the number of cells in the conceptus is small and their nature is not yet specialized, the effect of damage to these cells is most likely to take the form of a failure to implant or an undetected death of the conceptus. This is illustrated in the remarkable pictures produced by Pedersen and reproduced in Figure 12.3. If too many cells are killed by irradiation, the embryo dies and is resorbed. If only a few cells are killed, one or two cell divisions can make good the damage.
Organogenesis
During organogenesis, the principal effect of radiation in small rodents is the production of various congenital anomalies of a structural nature. As seen in Figure 12.1, a dose of about 2 Gy of x-rays to the mouse embryo during the period of maximum sensitivity can result in a 100% incidence of malformations at birth. A similar result is seen in Figure 12.2 for rats exposed to about 1 Gy. During organogenesis, most of the embryonic cells are in their blastula or differentiating stage and are particularly sensitive. This corresponds to the period in the human at which the tranquilizer thalidomide produced such disastrous effects (i.e., at about 35 days after conception), and it is also the time of maximum risk of deleterious effects from the rubella virus.
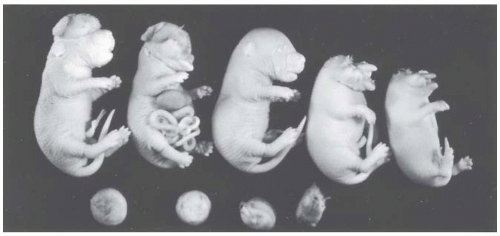
Examples of gross anomalies resulting from irradiation during the period of organogenesis are shown in Figures 12.4 and 12.5. It is characteristic of mice and rats that various structural malformations are seen. The production of a specific defect is associated with a definite time during this period of organogenesis, usually the time of the first morphologic evidence of differentiation in the organ or portion of the organ involved. On the basis of animal experiments, there appears to be a dose threshold of about 100 mGy for the induction of malformations during organogenesis, so that for practical purposes, the risks of
malformations in the human, for doses well below 100 mGy, would not be expected (ICRP 103).
malformations in the human, for doses well below 100 mGy, would not be expected (ICRP 103).
Embryos exposed during early organogenesis also exhibit the greatest intrauterine growth retardation. This is expressed as a weight reduction at term and is a phenomenon resulting from cell depletion. Animals show a remarkable ability to recover from the growth retardation produced by irradiation during organogenesis, and although they may be smaller than usual at birth, they may achieve a normal weight as adults. There is an association between growth retardation and teratogenesis: Irradiated embryos that show major congenital anomalies also suffer an overall reduction of growth. In animals, a dose of about 1 Gy of x-rays produces growth retardation if delivered at any stage of gestation (except during preimplantation); 0.25 Gy does not produce an observable effect, even at the most sensitive stage.
If death occurs because of irradiation in organogenesis, it is likely to be neonatal death— occurring at or about the time of birth. The transition from prenatal death from irradiation mainly during preimplantation to neonatal death resulting from irradiation mainly in organogenesis is very clear from Figure 12.1. The neonatal deaths peak at 70% for mice receiving about 2 Gy on about the 10th day.
The Fetal Period
The remainder of pregnancy, the fetal period, extends from about day 13 onward in the mouse; this corresponds to about 6 weeks onward in the human. Various effects have been documented in the experimental animal after irradiation during the fetal stages, including effects on the hematopoietic system, liver, and kidney, all occurring, however, after relatively high radiation doses. The effects on the developing gonads have been documented particularly well, both morphologically and functionally. There appears at present to be little correspondence between the cellular and functional damage as a function of dose, but doses of a few tenths of a gray as a minimum are necessary to produce fertility changes in various
animal species. The effects of radiation on humans were discussed in more detail in Chapter 11.
animal species. The effects of radiation on humans were discussed in more detail in Chapter 11.
Much higher doses of radiation are required to cause lethality during this period than at earlier stages of development, although the irradiated early fetus exhibits the largest degree of permanent growth retardation, in contrast to the embryo in early organogenesis, which exhibits the most temporary growth retardation, which is evident at term but from which the animal is able to recover later.
▪EXPERIENCE IN HUMANS
Information on the irradiation of human concepti comes from two major sources: studies of atomic bomb survivors in Japan and medical exposures (particularly therapeutic irradiations), especially during the early part of the century, when hazards were not yet fully appreciated. These will be discussed in turn.
Survivors of the A-Bomb Attacks on Hiroshima and Nagasaki Irradiated In Utero
The growth to maturity of children exposed in utero at Hiroshima and Nagasaki has been studied carefully. There are difficulties associated with the dosimetry, but the conclusions have far-reaching implications.
Data on the children exposed in utero in Hiroshima and Nagasaki show too few individuals who were younger than 4 weeks of gestational age at the time the bomb was dropped. This def ciency presumably results from increased fetal loss or infant mortality rate. This stage of development is so early that damage to a single cell or group of cells is likely to impair the function of all the progeny cells and leads to death of the embryo. In accord with this reasoning is the observation that no birth defects were found because of irradiation before 15 days of gestational age. This is in accord with the experimental data for rats and mice in which exposure during preimplantation had an all-or-nothing effect: death of the embryo or normal development.
Exposure to radiation resulted in growth retardation (Table 12.1). Children exposed as embryos closer than 1,500 m to the hypocenter of the atomic explosion were shorter, weighed less, and had head diameters significantly smaller than children who were more than 3,000 m from the hypocenter and received negligibly small doses. It is of interest to note that there was no catch-up growth because the smallness in head size was maintained into adulthood. The principal effects of irradiation in utero of the Japanese at Hiroshima and Nagasaki are small head size (microcephaly) and mental retardation. Figure 12.6 is one of the few photographs available of young Japanese adults who had been exposed in utero to radiation from the atomic bomb and whose head circumference is evidently smaller than normal. The prevalence of small head diameter and mental retardation has been evaluated in persons exposed in utero to the A-bombs in Japan. The study involved about 1,600 exposed children of whom about 62 had small heads and 30 showed clinically severe mental retardation.
TABLE 12.1 Growth Retardation at Hiroshima from In Utero Irradiation:a
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|
|---|