, Aaron C. Groen1 and Timothy J. Mitchison1
(1)
Department of Systems Biology, Harvard Medical School, Boston, MA, USA
Abstract
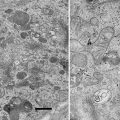
Cell-free cytoplasm isolated from meiotic Xenopus egg extracts reconstitutes microtubule phenomena in vitro. These crude extracts assemble bipolar meiotic spindles and are readily fractionated for biochemical assays, providing a good tool to dissect molecular mechanism. We developed techniques for immunoelectron microscopy of microtubule structures assembled in perfusion chambers and in solution.
Key words
Electron microscopyMicrotubulesPerfusion chambersCell-free cytoplasm1 Introduction
The cytoskeleton provides the architectural support for cell division. To better understand how the molecular components of the cytoskeleton function, we developed techniques to visualize the ultrastructure of microtubules assembled in meiotic Xenopus egg extracts using electron microscopy.
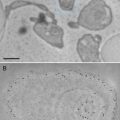
The crude meiotic extracts from unfertilized eggs of Xenopus laevis provide an excellent in vitro model for the recapitulation of cytoplasmic activities. One such activity, meiotic spindle assembly, requires precise organization of the microtubule cytoskeleton. The techniques we developed use immunoelectron microscopy to visualize meiotic spindles assembled in crude extract and microtubule structures induced with Taxol in fractionated extract. The fractionated extracts or clarified high-speed supernatant (C-HSS) are free of membranes and larger organelles, such as mitochondria, further facilitating direct visualization of the microtubule cytoskeleton. Our procedures provide opportunities to visualize the ultrastructure of microtubule structures and interacting molecules.
2 Materials
2.1 General Equipment
1.
Parafilm.
2.
Pipetman (P1000, P200, P20, P2) and appropriate tips.
3.
Aclar film for coverslips and mounting.
4.
Spearhead Power Punch and Die Set.
5.
Rubber mallet.
6.
Glow discharge apparatus.
7.
High molecular weight poly-l-lysine hydrobromide (Sigma).
8.
50-mL tri-pour beakers.
9.
250-mL polypropylene beakers.
10.
Ceramic coverslip holder with handle.
11.
Tongue depressor.
12.
Vacuum desiccator with desiccant.
13.
Polystyrene Petri dishes.
14.
Styrofoam shipping containers.
15.
Digital low-temperature thermometer.
16.
Fluorescence microscope.
17.
Dissecting microscope.
18.
Phase microscope.
19.
Polypropylene transfer pipettes.
20.
Polypropylene 15-mL round-bottom tubes with snap cap.
21.
Forceps.
22.
Heat block.
2.2 Equipment Specific for Xenopus Self-Assembled Microtubule Structures
2.
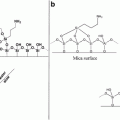
Perfusion chambers (see Fig. 1). 22 × 40-mm coverslips are cut from sheets of Aclar film (see Note 1). They are acetone cleaned, glow discharged and that surface incubated with a solution of 1 mg/mL poly-l-lysine. They are rinsed in distilled water and dried and then attached to a 1 × 3-inch glass microscope slide by daubing tiny spots of Velap on the corners of the nontreated surface, which is then liquefied on a 50 °C heat block until it spreads. Thin (7 mm) strips of Parafilm are stretched over the top and bottom of the Aclar coverslip leaving a space of approximately 8 mm between the inside edges. A passivated glass coverslip is placed treated side down on the Parafilm spacers. A P1000 pipet tip is used to gently press down on the coverslip to make a firm contact with the Aclar-Parafilm-glass sandwich. Warming the area with the Parafilm helps facilitate this. This perfusion chamber will hold a volume of 8–10 μL.
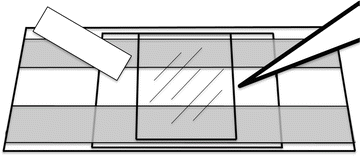

Fig. 1
Perfusion chamber for Xenopus egg extract HSS microtubule assembly reaction. The chamber is a 22 × 40-mm layer of Aclar attached to a glass microscope slide with melted Velap. Strips of Parafilm are stretched across the top and bottom of the Aclar. A 22 × 22-mm passivated glass coverslip is placed treated side down and pressed to the Parafilm with a 1,000-μL pipet tip. The HSS is introduced into one end of the chamber and wicked out from the other with filter paper
3.
Whatman 3 MM filter paper cut into 7 × 50-mm slivers.
4.
150-mm Petri dish humidification chamber.
2.3 Equipment Specific for Xenopus In Vitro Meiotic Spindle Experiments
1.
Spearhead Power Punch and Die Set.
2.
Rubber mallet.
3.
12.7-mm-diameter Aclar coverslips, glow discharged and coated with poly-l-lysine.
4.
Wide-bore (0.75–1 mm) P200 tips prepared by cutting them at the ends.
5.
Vacuum aspirator.
6.
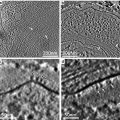
15-mL Kimble centrifuge tubes with removable chocks (see Fig. 2 and also [2]) that can be taken out using a large, unfolded paperclip. We mount a coverslip on top of the chock, onto which structures formed within extract can be centrifuged.
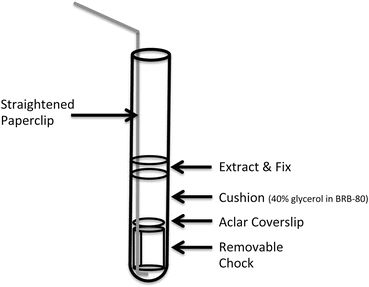

Fig. 2
Modified Corex tubes used to sediment spindles assembled in Xenopus egg extracts. A polycarbonate chock with a sidelong groove fits in the bottom of the tube and can be removed using a straightened, medium-sized paperclip. An Aclar coverslip is seated on top of the chock, which together are placed at the bottom of the tube. A glycerol cushion is overlaid onto the chock and coverslip, and fixed extract is pipetted over the glycerol cushion
2.4 Buffers
1.
5× BRB80: 400 mM K-PIPES, pH 6.8, 5 mM MgCl2, 5 mM K-ethylenebis(oxyethylenenitrilo)tetraacetic acid (K-EGTA) pH 7.7 [3].
2.
Dejelly buffer: (2 % cysteine in 100 mM KCl, 1 mM MgCl2, 0.1 mM CaCl2, pH 7.8).
4.
7.
HSS-XB egg washing buffer: (10 mM K-Phosphate, pH 7.7, 100 mM KCl, 1 mM MgCl2, 0.1 mM CaCl2) [5].
8.
Dilution buffer: (10 mM K-Phosphate, pH 7.7, 1 mM DTT, 1 mM ATP, 1 mM creatine phosphate, 1 mM GTP) [5].
9.
Cacodylate buffer: make 0.2 M solutions of sodium cacodylate trihydrate and 0.2 N HCl. Mix the two stocks to obtain the appropriate pH, then dilute with water to 50 mM.
10.
Calcium chloride: 40 mM stock.
2.5 Proteins, Protein Conjugates, and Drugs
1.
TPX antibody. Directly labeled with Alexa-647 succinimidyl ester. Affinity-purified C-terminal peptide antibody, as previously published [6].
2.
Glycogen.
3.
Rhodamine tubulin (directly labeled with carboxy-X-rhodamine (ROX), succinimidyl ester as previously described [7]).
4.
Protein A gold.
5.
Paclitaxel.
6.
S-trityl-l-cysteine (STLC).
2.6 Miscellaneous Reagents
1.




Free base lysine powder.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree