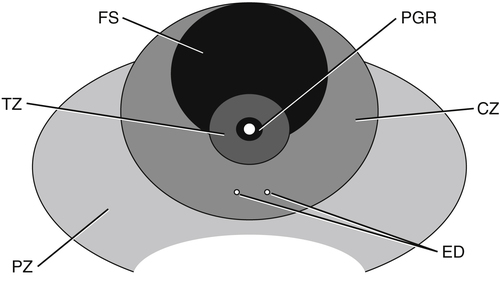
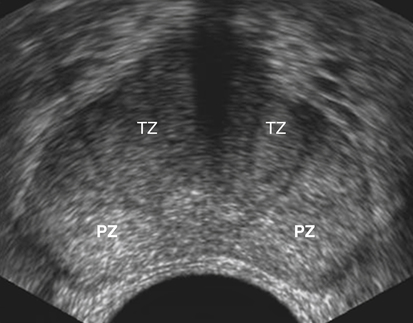
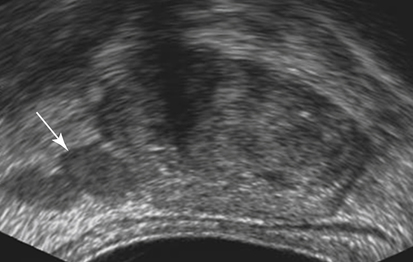
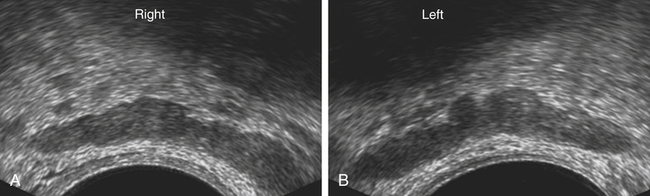
Dan J. Donovan and Diane J. Youngs • Describe the normal sonographic appearance of the prostate. • Describe the sonographic appearance of benign prostatic hypertrophy. • Describe the sonographic findings associated with prostatic inflammation. • Discuss the significance of sonographic evidence of calcifications and cystic areas within the prostate gland. • List clinical symptoms and sonographic findings associated with prostate cancer. • Discuss the role prostate specific antigen plays in the detection of prostate cancer and its limitations. Prostate carcinoma has become the most common cancer affecting North American men and is second only to lung cancer as a cause of cancer-related death. Age, race, ethnicity, and family history affect the risk for prostate cancer. Age is the main risk factor, and most men with clinically diagnosed prostate cancer are older than age 65 years.1 Because prostate cancer usually occurs at an age when conditions such as heart disease and stroke cause death, many men die with prostate cancer rather than because of it. Mortality rates from prostate cancer have decreased because of the use of new and combined treatments, improved imaging, and advances in biopsy techniques.2 The anatomic divisions of the prostate are referred to as zones, which have differing embryologic origins and susceptibilities to disease. There are four zones: the peripheral zone, transition zone, central zone, and anterior fibromuscular zone or stroma (Fig. 30-1). The outermost peripheral zone is the largest of the zones, it surrounds the distal urethral segment, and it is the site for most prostate cancers. The transition zone surrounds the proximal prostatic urethra and is most susceptible to benign prostatic hypertrophy (BPH). When hypertrophy is present, the transition zone may be the largest of the zones. The division between the peripheral and transition zones is called the surgical capsule. The central zone is a cone-shaped region that surrounds the ejaculatory ducts. This zone is rarely the site of prostate cancers and is thought to be relatively resistant to disease.3 Fibromuscular stroma is located anterior to the transition zone and prostatic urethra. The zonal anatomy can be difficult to differentiate sonographically. The peripheral and central zone make up the outer portion of the gland, and the inner gland consists of the transition zone, anterior fibromuscular stroma, and internal urethral sphincter. In young men, the predominant peripheral zone is poorly demarcated because it is isoechoic to the central and transitional zones. When the changes of BPH cause the transitional zone to become enlarged and hypoechoic, the peripheral zone appears relatively hyperechoic and is more easily recognized (Fig. 30-2). The peripheral and transition zones have a homogeneous echotexture; however, the transition zone may become heterogeneous when BPH is present. The surgical capsule is usually hypoechoic, unless corpora amylacea or calcifications have accumulated in this area. The vas deferens and seminal vesicles are visible superior to the base. The seminal vesicles are apparent as hypoechoic, oval, or tubular structures above the prostatic base (Figs. 30-3 and 30-4). Normal sonographic findings of the prostate in older men include dilated prostatic ducts (benign ductal ectasia); bright, echogenic foci or clumps representing calcifications; or proteinaceous debris (a precursor of calcific formation) called corpora amylacea. If no acoustic attenuation is present, these bright, echogenic areas may represent corpora amylacea. Calcifications and corpora amylacea are usually found in the periurethral glands and along the surgical capsule, although they can be found anywhere in the prostate (Figs. 30-5 and 30-6). Ductal ectasia may be evident in the peripheral zone. These findings are indicative of a benign process. The decision to perform TRUS is typically based on the result of a PSA test and a DRE. Table 30-1 lists the components of a typical sonographic evaluation of the prostate. TABLE 30-1 Sonographic Evaluation of Prostate Imaging of prostate in two planes (sagittal and transverse) Evaluation of gland for size, echogenicity, and symmetry Evaluation of periprostatic fat and vessels for asymmetry Examination of seminal vesicles in two planes for size, shape, position, symmetry, and echogenicity Evaluation of vas deferens Survey of perirectal space with attention to rectal wall and lumen PSA is a protein produced by the cells of the prostate gland. The PSA test measures the level of PSA in the blood and is considered a valuable screening tool for early, curable, organ-confined prostate cancer. On a volume-per-volume basis, prostate cancer contributes 10 times the amount of PSA to the serum as benign prostate tissue, which is why serum PSA levels are often elevated in patients with prostate cancer.3 PSA levels also increase with tumor volume and stage. This increase is not the result of increased production of PSA; rather, it is the result of increased diffusion (leaking) of PSA into the serum. The more aggressive the tumor, the more disruption of prostatic architecture and the higher the PSA level. Earlier accepted standards placed the normal range for total PSA at 0.0 to 4.0 ng/mL, and it was believed if the PSA level was less than 4.0 ng/mL, a biopsy was not needed. This threshold was adjusted to 0.0 to 2.5 ng/mL; however, a more recent study suggested that both white and African American men with baseline PSA values between 1.5 ng/mL and 4.0 ng/mL are at increased risk for future prostate cancer.4,5 Because using a specific PSA cut-point can lead to an overestimation of risk in some patients and underestimation in others, the American Urological Association published recommendations that biopsy decisions not only should be based on the results of PSA testing and DRE but also take into account free and total PSA, patient age, PSA velocity, PSA density, family history, ethnicity, prior biopsy history, and comorbidities.6 PSA density considers the relationship of the PSA level to the size of the prostate. PSA density is calculated by dividing the PSA level by the volume of the prostate gland determined during TRUS examination (PSA density = serum PSA/prostate volume). In other words, an elevated PSA level in a small gland may be suspicious, whereas an elevated PSA in a larger gland would be considered less suspicious. A PSA density of 0.1 or less is considered normal, a PSA density of 0.10 to 0.15 is intermediate, and a PSA density greater than 0.15 is considered elevated.3 PSA velocity is based on changes of PSA levels over time. A sharp increase in PSA level (>20% per year) is worrisome for malignant disease. Three PSA values over a period of at least 18 months is recommended to establish PSA velocity.6
Elevated Prostate Specific Antigen
Prostate Anatomy
Sonographic Appearance
Normal Variants
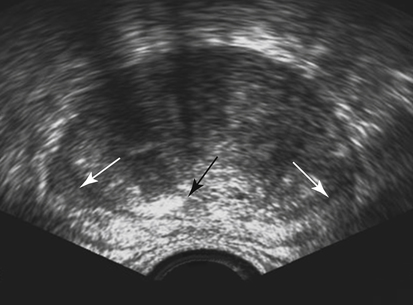
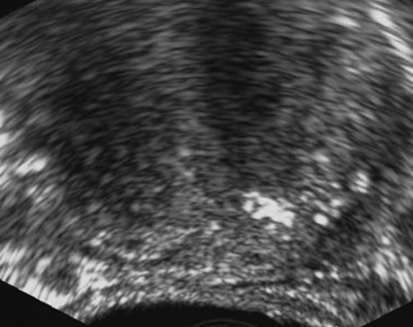
Prostate Specific Antigen
Prostate Specific Antigen Density
Prostate Specific Antigen Velocity
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine