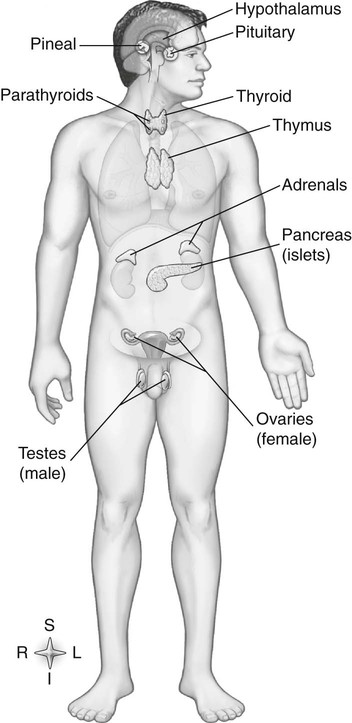
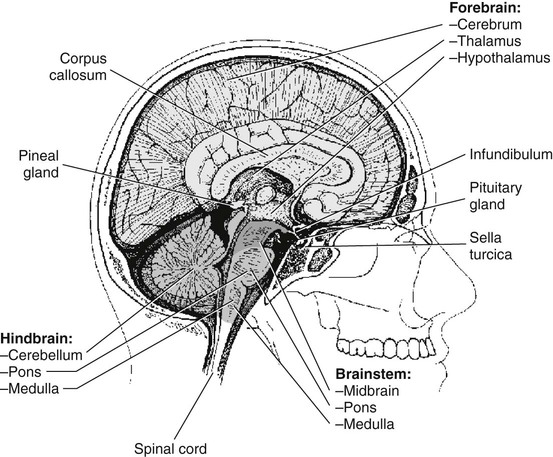
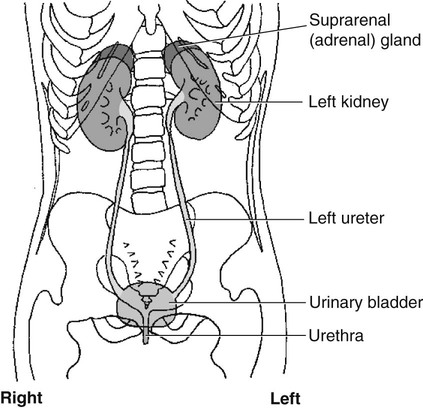
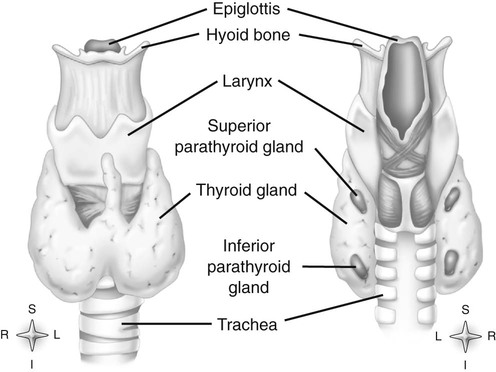
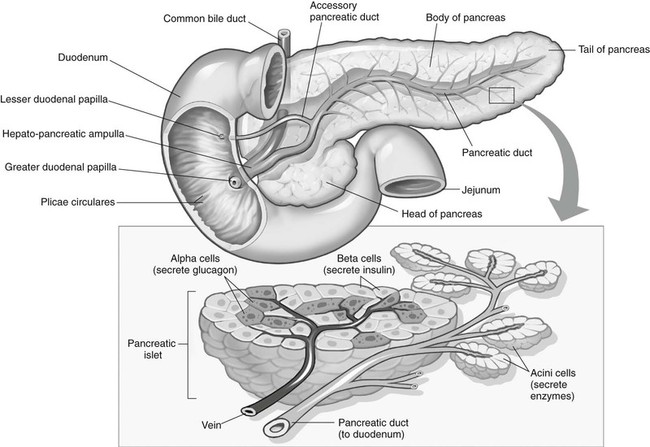
Chapter 11 On completion of Chapter 11, the reader should be able to: • Describe the anatomic components and function of the endocrine system. • Identify and explain the various imaging modalities used in diagnosing and treating endocrine disorders. • Specify the disorders, signs and symptoms, and prognosis of the endocrine pathologies discussed in this chapter. The endocrine system is responsible for metabolic activities within the human cells through the release of hormones. Endocrine glands are ductless glands that secrete hormones directly into the surrounding vascular and lymphatic systems or through neuroendocrine processes. The primary endocrine glands of the body include the pituitary gland, pineal gland, adrenal glands, thyroid gland, parathyroid gland, and thymus gland. The pancreas, gonads, and hypothalamus also produce hormones (Fig. 11-1). Hormones are synthesized and released in response to three types of stimuli: (1) humoral stimuli, which involve a direct response to changes in blood chemistry; (2) neural stimuli, such as in the case of the adrenal glands secreting epinephrine or norepinephrine in response to the sympathetic nervous system; and (3) hormonal stimuli, which involve a response to other hormones secreted in the body. The pituitary gland comprises three separate lobes: (1) the anterior lobe, (2) the intermediate lobe, and (3) the posterior lobe. Each lobe functions as a separate gland; however, in humans, the intermediate lobe is rudimentary, consisting of only a few cells. The pituitary is considered the “master endocrine gland,” and it is located below the hypothalamus at the base of the brain within the sella turcica of the sphenoid bone (Fig. 11-2). The hypothalamus is connected to the pituitary via the pituitary stalk, and the hypothalamus is responsible for controlling the function of the pituitary gland. Each lobe of the pituitary secretes specific hormones. The anterior lobe is responsible for secreting luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin (PRL), adrenocorticotropic hormone (ACTH), growth hormone (GH), and thyroid-stimulating hormone (TSH). The intermediate lobe secretes melanocyte-stimulating hormone (MSH), and the posterior lobe is responsible for secreting oxytocin and antidiuretic hormone (ADH). The secretion of hormones by the anterior lobe occurs through the typical endocrine control mechanism by the release of hormones directly into the blood. However, the posterior lobe of the pituitary is an extension of the nervous system, with hormone secretion occurring via a neuroendocrine mechanism, in which the hormones are synthesized within the cell bodies of neurons within the hypothalamus. The pineal gland is also controlled by the hypothalamus. It is located within the cranium, posterior to the third ventricle, superior to the colliculi of the midbrain, and inferior to the splenium of the corpus callosum (see Fig. 11-2). It occasionally calcifies with age and is often quite visible on computed tomography (CT) images of the brain. This small endocrine gland is responsible for the production and excretion of melatonin. Melatonin is released when light exposure is inhibited; thus, the pineal gland is responsible for regulating the circadian rhythms. This hormone is often used to treat sleep disorders. The pineal gland also secretes gonadotropin-releasing hormone (GnRH), a hormone that stimulates the release of FSH and LH and is active in the onset of puberty. The adrenal glands are pyramid-shaped glands located on the upper poles of the kidneys (Fig. 11-3). The inner portion of the adrenal gland, termed the adrenal medulla, acts as a part of the sympathetic nervous system and secretes the catecholamines epinephrine and norepinephrine. The effects of increased epinephrine production include increased cardiac output; increased heart rate; vasodilation of skeletal muscle; vasoconstriction of internal organs and skin; relaxation of smooth muscle in the gastrointestinal (GI) system, urinary bladder, and bronchials; and increased mental alertness. Epinephrine also increases glycogenesis in the liver and lipolysis in adipose tissue, as well as affecting the pancreatic hormone secretion by decreasing insulin production and increasing glucagon secretion. The outer portion, known as the adrenal cortex, is responsible for corticosteroid production. The adrenal cortex activity is primarily controlled by ACTH, which is secreted by the anterior pituitary. ACTH synthesizes three major types of corticosteroids: (1) mineralocorticosteroids, which are responsible for electrolyte balance; (2) glucocorticoids, which are responsible for cell metabolism and for controlling blood sugar levels; and (3) low levels of gonadocorticosteroids or sex hormones. Mineralocorticoids such as aldosterone may affect extracellular fluid volume and blood volume, which ultimately affects blood pressure and cardiac output. Glucocorticoid secretions are greatly influenced by physical or psychological stress stimuli such as hypoglycemia, trauma, and acute or chronic anxiety. Hypoglycemia stimulates the secretion of cortisol, which, in turn, triggers a response in the liver to convert amino acids to glucose, thus increasing blood glucose levels. The thyroid gland is located in the anterior neck just below the larynx (Fig. 11-4). It is divided into two lobes connected by an isthmus. This endocrine gland secretes two hormones: (1) thyroid hormone (TH) and (2) calcitonin. TH stimulates enzymes responsible for glucose oxidation and is actually a composition of two hormones: (1) thyroxine (T4) and (2) triiodothyronine (T3), both of which contain iodine as an integral part of the molecule. Unlike other hormones within the body, the thyroid hormone synthesis requires the presence of iodine. Levels of T4 and T3 are regulated by TSH, which is synthesized and stored within the anterior lobe of the pituitary. The thyroid gland is vital to maintaining normal blood pressure and in regulating tissue growth and development. Overactivity of the thyroid gland is termed hyperthyroidism, and underactivity is termed hypothyroidism. Either abnormal activity level may cause severe metabolic disturbances in the human body. Calcitonin is most important in childhood. It is an antagonist of parathyroid hormone (PTH) and serves to lower blood calcium levels by inhibiting osteoclast activity and stimulating calcium uptake in the bone matrix. The parathyroid glands are located on the posterior aspect of the thyroid gland. They are responsible for producing and secreting PTH, which also serves to control blood calcium levels. This hormone stimulates osteoclasts and increases calcium absorption in the kidneys and in the GI system. It is important to note that a reduction in bone density may occur and result in increased bone fragility if PTH secretion is sustained at a high level. However, new research in the use of therapeutic recombinant parathyroid hormone (rPTH) shows promise in strengthening bone and increasing bone density in patients with osteoporosis. The pancreas is located in the midabdomen just posterior to the stomach (Fig. 11-5) and functions both as an endocrine organ and an exocrine organ. The tail of the pancreas ends at the spleen, and the head is encircled by the duodenum. Specialized cells within the pancreas, termed the islets of Langerhans or pancreatic islets, are responsible for the production of important hormones. The α-cells are responsible for glucagon synthesis; the β-cells are responsible for balancing the secretion of insulin and glucagon, depending on food intake; and the δ-cells are responsible for secreting somatostatin, a hormone which influences the secretion of the α-cells and β-cells. Glucagon promotes the breakdown of glycogen to glucose within the liver, and glucose is ultimately released into the bloodstream. The α-cells of the pancreas secrete glucagon to increase blood sugar levels, and these cells are driven by humeral stimuli. Insulin, whose role is the opposite of glucagon’s, acts to decrease blood sugar levels. Insulin is produced by the β-cells to inhibit the breakdown of glycogen to glucose. MRI has largely replaced CT for imaging neuroendocrine disorders. According to the American College of Radiology (ACR), MRI is the only imaging modality that can reliably demonstrate pathologies of the hypothalamus. Pituitary disorders often result in an enlarged sella turcica, represented by the “empty sella syndrome,” which can be confirmed with MRI. Gadolinium contrast-enhanced MRI is routinely used to demonstrate pituitary adenomas and is the modality of choice for visualizing microadenomas. MRI is also helpful in monitoring the progress of patients with pituitary adenomas. (See Chapter 8 for additional information on pituitary adenomas.) A radiographically visible decrease in bone density is termed osteopenia and may occur as a result of osteoporosis or osteomalacia. Osteopenia is identified as a bone mass between 648 and 833 milligram per square centimeter (mg/cm2). Osteoporosis is a commonly known metabolic bone disorder in which the structural integrity of the trabecular pattern of bone is destroyed and is identified as a bone mass less than 648 mg/cm2. It may be classified as primary osteoporosis (type 1) or secondary osteoporosis (type 2). Primary osteoporosis may be further classified as postmenopausal or senile, and secondary osteoporosis is most commonly associated with an existing disease process or is the result of a medication. Osteoporosis may also be classified as generalized or regional. Postmenopausal osteoporosis is the most common form of the disease. Estimates are that more than half of the women in North America over age 60 years have osteoporosis, and it is a major cause of fractures of the hip, spine, and wrist in women over age 50 years. It is more common in white and Asian women. Although the formation of bone is normal, the bone reabsorption rate is abnormally high in individuals with osteoporosis. Thus, osteoporosis results in a thinning of cortical bone and an enlargement of the medullary canal without any change in the actual diameter of the bone. The normal equilibrium associated with osteoid production and withdrawal is quite complex and depends on a combination of dietary intake and absorption, hormonal interplay, and normal stress or muscular activity. In postmenopausal women, for example, the lack of the hormone estrogen creates a weakened bone matrix, contributing to the development of “porous” bones. As the condition becomes more severe, bones are subject to compression fractures, and they may literally cave in from the weakness. Risk factors for osteoporosis are listed in Box 11-1
Endocrine System
Anatomy and Physiology
Imaging Considerations
Radiography
Magnetic Resonance Imaging
Skeletal Disorders
Osteoporosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine