Disorder
Endovascular therapy available?
Catheter-directed medical treatment
Revascularization
Vasodilator
Fibrinolysis
Chronic intestinal ischemia
Atherosclerotic
X
Arcuate ligament syndrome
+/−
Rare vascular causes
X
Mesenteric venous thrombosis
X
Acute intestinal ischemia
Thromboembolism
X
X
X
Arterial thrombosis
X
X
X
Nonocclusive
X
Venous thrombosis
X
This chapter focuses on the percutaneous treatment of acute and chronic mesenteric ischemia, in the context of a complementary, multidisciplinary approach to these diagnostically and therapeutically challenging disorders. Comprehensive endovascular therapy is reviewed, including patient selection, technical aspects of intervention, catheter-directed delivery of vasodilators and fibrinolytics, percutaneous revascularization, concomitant medical therapy, and follow-up considerations. Relevant anatomic, pathophysiologic, and clinical information is also summarized.
Mesenteric Arterial Anatomy for the Interventionalist
The intestinal blood supply arises from three major branches of the abdominal aorta: the celiac, superior mesenteric, and inferior mesenteric arteries (Fig. 33.1). Each vessel arises anteriorly from the aorta. Typically, the celiac artery has a horizontal takeoff, while the SMA takes a downward course [11, 12]. Appreciation of the level, trajectory, of the origin of these vessels, as well as their caliber and branches, influences the selection of guiding catheters, positioning of guide wires, and choice of angioplasty balloons and stents (Table 33.2).
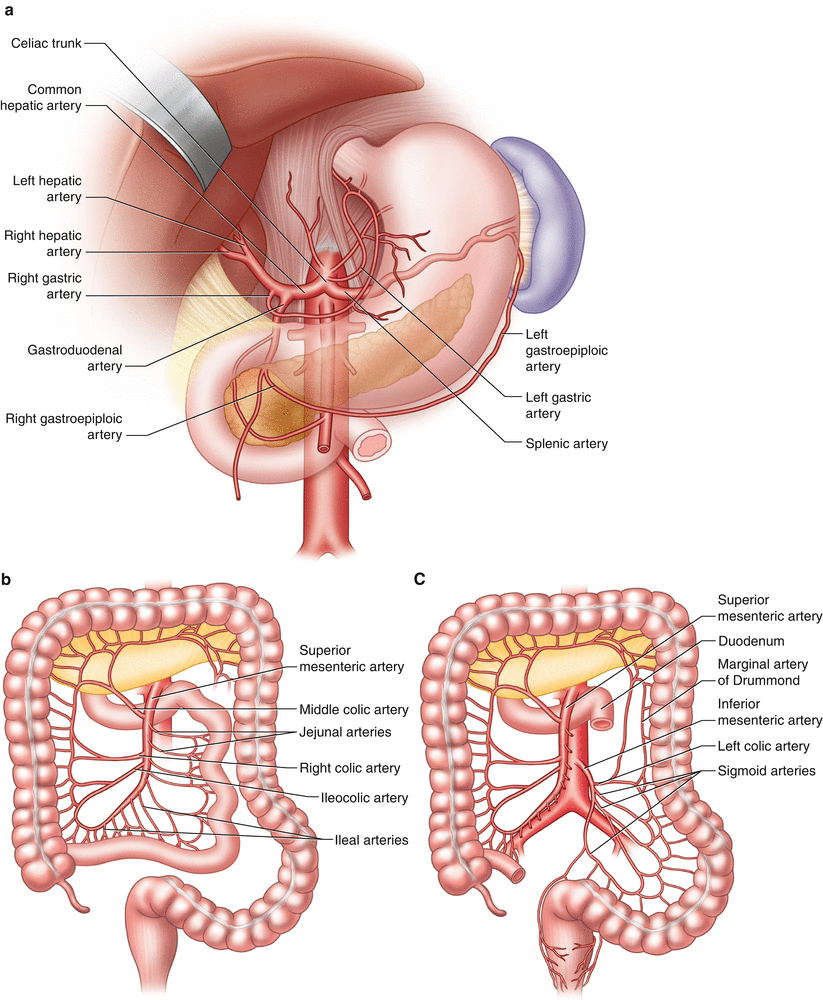

Fig. 33.1
Anatomy of (a) celiac, (b) superior mesenteric, and (c) inferior mesenteric arteries and branches
Table 33.2
First-order mesenteric branches
Level of takeoff | Diameter | Major branches | Organs supplied | |
|---|---|---|---|---|
Celiac trunk | T12 | 5–7 mm | Common hepatic artery | Stomach |
Splenic artery | Duodenum | |||
Left gastric artery | Spleen | |||
Liver | ||||
Pancreas | ||||
SMA | L1 | 4–6 mm | Inferior pancreaticoduodenal | Pancreas |
1.5 cm below celiacartery | Middle colic artery | Jejunum | ||
Right colic artery | Ileum | |||
Ileocolic artery | Ascending colon | |||
Jejunal arteries | Proximal half of transverse colon | |||
Ileal arteries | ||||
IMA | L3 | 3–5 mm | Left colic | Distal half of transverse colon |
4 cm above aortoiliacbifurcation | Sigmoid | Descending colon | ||
Marginal | Rectum | |||
Superior rectal |
The celiac trunk is typically a 5–7 mm vessel and arises at the level of the 12th thoracic vertebra [11–13]. It gives rise to the common hepatic and splenic arteries and often the left gastric artery. Any of these branches may arise directly from the aorta, however. The celiac axis supplies the stomach, duodenum, spleen, liver, and pancreas (Table 33.2).
The SMA is 4–6 mm in caliber and arises at the level of the 1st lumbar vertebra, 1.5 cm below the origin of the celiac trunk [11, 13]. Its branches include the inferior pancreaticoduodenal, middle colic, right colic, ileocolic, jejunal, and ileal arteries. Variations of this anatomy include the SMA or its colic branches arising from the celiac trunk. The SMA system supplies the pancreas, jejunum, ileum, ascending colon, and proximal half of the transverse colon (Table 33.2).
The IMA is 3–5 mm in diameter [11, 13] and arises at the level of the 3rd lumbar vertebra, approximately 4 cm above the aortoiliac bifurcation. Its branches include the left colic, sigmoid, marginal, and superior rectal arteries, which have important anastamoses with the inferior rectal arteries of the internal iliac arteries. The IMA system supplies the distal half of the transverse colon, descending colon, and rectum (Table 33.2).
The development of chronic mesenteric ischemia is usually the result of multivessel disease [4, 7, 8, 12]. This phenomenon is due to the typical ostial location of atherosclerotic lesions and to the presence of multiple, more distal anastomotic connections between the mesenteric vessels (Table 33.3) [11–13]. In contrast, acute mesenteric ischemia most often involves a single vessel, the SMA. Embolization has a predilection for this vessel likely due to its acute, downward-going takeoff from the aorta [12]. A common location of SMA embolization is just distal to the takeoff of the middle colic artery, which compromises multiple pathways of anastomotic supply to the SMA territory – the marginal artery of Drummond, arc of Riolan, and pancreaticoduodenal arcade [12] (Table 33.3).
Table 33.3
Major anastamoses in mesenteric arterial circulation
Anastomosis | Anatomy | Major branches connected | Clinical relevance |
|---|---|---|---|
Inferior phrenic arteries | Inferior phrenic arteries–hepatic arteries | Aorta and celiac axis | Celiac occlusion |
Arc of Barkow | Hepatic/gastroduodenal/right gastroepiploicarteries–left gastroepiploic/splenic arteries | Celiac–celiac axis | |
Pancreaticoduodenalarcade | Common hepatic/gastroduodenal/superiorPD arteries–inferior PD artery | Celiac and SMA | Celiac or proximal SMA occlusion |
Arc of Buhler | Persistent embryonic connection | Celiac and SMA | 4 % occurrence |
Marginal artery of Drummond | Confluence of right and middle colicarteries (SMA) with left colic artery (IMA) | SMA and IMA | Proximal SMA occlusion |
Arc of Riolan | Middle colic (SMA)–left colic artery (IMA) | SMA and IMA | Proximal SMA occlusion |
Rectal arteries | Superior rectal artery (IMA)–middleand inferior rectal arteries (IIA) | IMA and IIA | Celiac/SMA/IMA occlusions |
Clinical Syndromes
Chronic Mesenteric Ischemia
Chronic intestinal ischemia results from inadequate oxygen supply to the gut during digestion. 10 % of cardiac output perfuses the intestines at rest, while up to 35 % is required following a meal [14]. This hyperemic response is directed primarily to the small intestine and pancreas, which are supplied by the SMA and its branches; hence, the SMA is the most frequently diseased mesenteric artery in patients manifesting symptoms of chronic mesenteric ischemia [14, 15].
The incidence of chronic mesenteric ischemia is estimated at 1/100,000 per year in the general population [6]. The most common cause of chronic mesenteric ischemia is atherosclerosis, which affects an older patient population with a slight female preponderance [15]. Over 30 % of patients with chronic intestinal ischemia due to atherosclerosis have concomitant atherosclerotic disease of other vascular beds, and 75 % are smokers [15]. More rare etiologies of chronic mesenteric ischemia include fibromuscular dysplasia and several vasculitides.
The classic presenting symptom of chronic mesenteric ischemia has been described as “abdominal angina,” which is a cramping discomfort occurring anywhere from 15 min to 3 h after a meal [16]. Patients develop an aversion to food (sitophobia) resulting in weight loss over the months prior to presentation. In fact, the diagnosis should be questioned in the absence of weight loss. Other symptoms may include nausea, vomiting, and changes in bowel habits [4, 16]. On exam, a systolic bruit may be heard as well as signs of atherosclerosis in other arterial distributions.
Chronic Mesenteric Ischemia due to Atherosclerotic Disease
It is for this entity that percutaneous treatment of mesenteric vascular disease is most frequently employed. As mentioned, due to the various anastomotic pathways (Table 33.3) that allow single-vessel disease to be well tolerated, chronic intestinal ischemia most commonly results from multivessel disease of the mesenteric vessels. In nine reports of 244 cases of chronic mesenteric ischemia published in the 1980s and 1990s, 91 % of cases involved 2-vessel disease, 55 % involved 3-vessel disease, and 9 % had single-vessel disease (7 % SMA, 2 % celiac) by catheter-based angiography [15]. Though uncommon, single-vessel mesenteric disease can result in symptomatic chronic intestinal ischemia in the presence of insufficient collateral reserve. Individuals with prior abdominal surgery are particularly susceptible to disrupted collateral supply. Culprit atherosclerotic lesions most commonly affect the ostium of the mesenteric vessels and are frequently heavily calcified.
Several imaging modalities facilitate the diagnosis of chronic mesenteric ischemia due to atherosclerosis and have largely replaced the need for diagnostic invasive angiography for this disease. Doppler ultrasonography is an excellent modality in the diagnosis of obstructive atherosclerotic disease of the celiac and superior mesenteric arteries provided the study is performed by a skilled technician and the patient’s body habitus is not prohibitive [15, 17]. Due to the proximal nature of typical atherosclerotic stenoses, computed tomographic angiography and magnetic resonance angiography are also accurate imaging tools. Though superior in its imaging resolution, catheter-based angiography is not a first-line diagnostic test for chronic intestinal ischemia due to a relatively higher rate of vascular complications than other imaging tests and due to the exposure to iodinated contrast and radiation as compared to duplex ultrasonography or magnetic resonance angiography. It should be emphasized that the presence of obstructive lesions on any of these modalities is not sufficient to make the diagnosis of chronic intestinal ischemia; rather, the presence of suggestive clinical symptoms is also necessary. Thus, catheter-based angiography – with an inclination for endovascular treatment – is typically reserved for the patient with suggestive symptoms of chronic mesenteric ischemia in addition to evidence of severe obstructive disease on a less-invasive imaging modality.
Asymptomatic mesenteric atherosclerosis is thought to have a relatively high prevalence but a benign natural history. In a population-based study of 553 elderly individuals, the prevalence of single-vessel mesenteric obstructive disease was 17 % by duplex ultrasonography [18]. The favorable prognosis of asymptomatic mesenteric atherosclerotic disease is largely based on a report from the Cardiovascular Health Study; however, this report suffered from incomplete follow-up [18]. Nonetheless, it is generally accepted that medical therapy, in the absence of revascularization, is warranted for asymptomatic patients [6].
For patients with symptoms and weight loss thought to be due to obstructive, atherosclerotic mesenteric disease, revascularization is warranted. No randomized trials have been conducted comparing the various treatment options of chronic intestinal ischemia due to atherosclerotic disease. Surgical revascularization was 1st done in 1958 [19]. Options include bypass, endarterectomy, and reimplantation. Bypass choices include single-vessel versus multivessel revascularization, prosthetic versus venous conduits, and antegrade (from the supraceliac aorta) versus retrograde (from the internal iliac arteries) approaches [15]. Depending on the study, perioperative mortality has ranged from 0 to 16 %, and reported recurrence rates have varied widely from 0 to 26 % over 3–12 years [8].
Feasible, percutaneous revascularization is generally preferred over surgical revascularization due to the morbidity associated with surgery in the typically elderly population with this disease. However, certain patient subsets are more appropriate for surgery, including those with concomitant aneurysmal disease in need of repair and those with flush occlusions of the culprit mesenteric vessel(s) [6]. The technical aspects of endovascular therapy and its evidence base are discussed later in this chapter.
Nonatherosclerotic Causes of Chronic Mesenteric Ischemia
Less than 10 % of cases of chronic intestinal ischemia are due to nonatherosclerotic diseases, which are summarized in Table 33.4 [16]. In general, catheter-based angiography is employed more frequently for the diagnosis of fibromuscular dysplasia and vasculitis than for atherosclerotic disease due to involvement of mid and distal mesenteric vessels by these nonatherosclerotic diseases and consequently the need for an imaging modality with higher resolution. There is minimal literature to assist with decision-making regarding revascularization for chronic intestinal ischemia in fibromuscular dysplasia and vasculitis. In the absence of such evidence, extrapolation of data from intervention in other arterial beds seems appropriate. Thus, for chronic intestinal ischemia due to fibromuscular dysplasia, measurement of pressure gradients to assess severity of stenoses and angioplasty alone without stenting should be considered as is done for renal artery interventions for this disorder [20]. Also, endovascular therapy for chronic mesenteric ischemia due to vasculitis should be performed in the chronic fibrotic stage, rather than the active inflammatory stage, to improve durability of the intervention.
Table 33.4
Nonatherosclerotic, nontraumatic causes of chronic intestinal ischemia
Demographic | Noninvasive imaging | Catheter-based angiographic findings | Endovascular therapy | |
|---|---|---|---|---|
Fibromuscular dysplasia | Most commonly20–40-year-old women | Mid-distal lesions may be difficult to visualize. “String of beads” for medial fibroplasias. Long smooth stenoses if intimal fibroplasia. No vessel wall edema as seen in vasculitis | “String of beads” for medial fibroplasia. Long smooth stenoses if intimal fibroplasia. Cause of spontaneous dissection | PTA after assessment with IVUS/pressure gradient with “bailout” stenting |
Segmental arterial mediolysis | >40 years | Aneurysmal disease | Aneurysmal disease | No reported cases |
Vasculitis | Depends on type | Mid-distal lesions difficult to visualize. MRI and US may show wall edema. Proximal stenoses may be seen in large-vessel vasculitis | Large-vessel vasculitis (e.g., Takayasu’s): Smooth stenoses of proximal mesenteric vessels | For large-vessel vasculitis, PTA/stenting rarely, in chronic fibrotic stage of disease. Not recommended in acute inflammatory stage |
Medium vessel vasculitis (PAN): multiple aneurysms <1 cm +/− stenoses in distal medium-sized branches of mesenteric vessels | ||||
Radiation induced | History of >4,500 cGyabdominal radiation | Mid-distal lesions difficult to visualize | Multiple stenoses of small vessels | No reported cases |
Median arcuate ligament syndrome | Typically, youngthin females | US: elevated velocities in proximal celiac trunk during expiration | Rarely necessary for diagnosis | Stenting – controversial |
CT: “hooked” appearance of proximal celiac trunk | Respirophasic proximal celiac trunk stenosis (worse in expiration) |
Median arcuate ligament syndrome is a controversial entity that refers to compression of the celiac trunk by the arcuate ligament of the diaphragm in the presence of symptoms of chronic mesenteric ischemia and in the absence of alternative etiologies. The stenosis is dynamic, becoming more severe in expiration when the diaphragm moves upward. Duplex ultrasonography shows elevated peak systolic velocities during expiration. CT angiography shows a focal, ostial narrowing with an acutely downward-going proximal segment forming a classic “hooked” appearance [21, 22].
Celiac artery compression by the arcuate ligament occurs not infrequently in the absence of symptoms [23]. Thus, establishing a cause-and-effect relationship between this finding and symptoms is challenging and likely only possible if symptoms improve after revascularization. However, given the uncertain benefit, the indication for revascularization is often suspect. If revascularization is pursued, surgical release of compression with or without celiac trunk revascularization is recommended [6]. Laparoscopic release with or without endovascular revascularization has been described as a minimally invasive option ([24]. It is important for the interventionalist not to mistake this syndrome with atherosclerotic disease of the celiac artery.
Acute Mesenteric Ischemia
Patients with acute intestinal ischemia have a 60–80 % mortality rate [10]. Outcomes can be improved if prompt diagnosis leads to efficient mesenteric reperfusion such that viable bowel can be preserved, the extent of necrotic bowel can be limited, and necrotic intestine can be resected [10, 25].
Surgery – explorative laparotomy, bowel resection, and revascularization – is a mainstay of the management of acute intestinal ischemia [4, 8, 10]. In contemporary medicine, however, multiple endovascular treatments are also available [26–29]. When used in conjunction with surgery, endovascular techniques can optimize prompt and efficient diagnosis and treatment of patients with acute intestinal ischemia.
Given the high mortality rate, challenging diagnosis, and varied treatment options spanning multiple specialties, efficient multidisciplinary collaboration is essential in achieving optimal patient outcomes.
Etiology
Acute mesenteric ischemia can result from arterial occlusion, nonocclusive mesenteric ischemia, or venous thrombosis. Arterial occlusion most commonly results from thromboembolism, which accounts for 30–50 % of cases of acute mesenteric ischemia. In situ thrombosis is another common cause of arterial occlusion, contributing to 25–30 % of cases of acute mesenteric ischemia. Less common causes of acute mesenteric arterial occlusion are atheroembolism and dissection [5, 10, 14]. Nonocclusive mesenteric ischemia (NOMI) refers to a state of low cardiac output accompanied by profound mesenteric vasoconstriction in the absence of focal mesenteric arterial obstruction. This entity is responsible for 20–30 % of acute mesenteric ischemia cases [10]. It can also be due to intense mesenteric vasospasm from ergotamines, digoxin, cocaine, amphetamine, or vasopressor agents without reduced cardiac output [4]. Venous thrombosis of the celiac and/or superior mesenteric veins is typically seen in conditions predisposing to deep venous thrombosis – such as protein C deficiency, protein S deficiency, factor V Leiden, and prothrombin gene mutation – and is reported to account for 15–20 % of acute intestinal ischemia [10, 30]. Other risk factors for mesenteric venous thrombosis include pancreatitis, recent intra-abdominal surgery, and cirrhosis [30].
Presentation
Regardless of etiology, patients with acute mesenteric ischemia present with severe abdominal pain that is classically out of proportion to physical findings. Other symptoms include nausea, vomiting, diarrhea, and gastrointestinal bleeding. On physical exam, a rigid abdomen indicates peritonitis from perforation of necrotic bowel. Such a finding is critical in the early evaluation of this disease as it indicates the need for emergent surgery, the absence of which carries a high mortality rate [4]. Melena may also be present, though the absence of heme-positive stools cannot rule out acute intestinal ischemia [4]. Lactic acidosis is common, but an elevated lactate is nonspecific.
In all comers with acute intestinal ischemia, the median age is 70 years and 2/3 are women [4]. There may be clinical features that suggest a precise etiology in patients presenting with acute mesenteric ischemia, and recognition of these clues can facilitate appropriate initial workup and management. For example, potential sources of thrombotic or atherosclerotic embolism should be sought (e.g., atrial fibrillation, severe left ventricular dysfunction, aortic atheroma, and a recent arterial catheterization procedure), particularly in the nonanticoagulated patient. These patients may also have evidence of embolization elsewhere, causing acute stroke or acute limb ischemia. A history of chronic intestinal ischemic symptoms suggests in situ atherothrombosis as the etiology of acute intestinal ischemia. Critical illness, hypotension from any cause, and certain offending medications (vasopressors, digoxin, ergotamines) are risk factors for NOMI. Finally, a known hypercoagulable state and pancreatitis may be present in patients with mesenteric venous thrombosis.