Treatment of arteriovenous malformations of the central nervous system requires a multidisciplinary approach with input from vascular neurosurgeons, endovascular interventionalists, and radiation oncologists. Treatment paradigms based on a thorough understanding of the natural history of the lesion and the cumulative risks of multimodality treatment maximize the likelihood of a positive outcome. This article outlines the role of endovascular embolization in the treatment of arteriovenous malformations with specific emphasis on decision making during treatment planning. Technical considerations when treating arteriovenous malformations are discussed, including the choice of embolic agents, potential intraprocedural and periprocedural complications, and postprocedural management of patients.
Key points
- •
In the year after a symptomatic hemorrhage from a cerebral arteriovenous malformation (AVM), the rate of hemorrhage is believed to be higher, in the order of 6% to 18% per year, returning to the 2% to 4% baseline over time.
- •
After an AVM ruptures, the risk of death is 10% and the risk of major disability is 20% to 30%.
- •
The operative morbidity and mortality rates are 1% and 3% for Grade I/II and Grade III cerebral AVMs, respectively.
- •
The morbidity and mortality rates in the immediate postoperative period associated with treating Grade IV and V cerebral AVMs may be as high as 31% and 50%, respectively.
- •
Grade IV and V cerebral AVMs are treated only in patients with progressive neurologic deficits attributable to repeated hemorrhage or disabling symptoms, such as intractable seizures.
- •
Current data suggest that partial treatment of cerebral AVMs increases the risk of future hemorrhage.
- •
In select patients with Grade IV and V AVMs, partial treatment targeted to eliminate an identified bleeding source (ie, intranidal aneurysm) may be undertaken.
- •
Embolization can be used to reduce the size of the AVM nidus to a range that is amenable to radiosurgical ablation.
- •
A small cerebral AVM with a limited number of feeding pedicles can be cured completely using endovascular embolization with a permanent agent (eg, n -butyl cyanoacrylate [NBCA] or Onyx, not particles).
- •
NBCA and Onyx are the primary liquid embolic agents used for endovascular treatment of cerebral AVMs.
- •
Flow-related aneurysms proximal to the nidus (feeding vessel or circle of Willis) and nidal aneurysms should be addressed before embolization of the AVM.
- •
A staged embolization for large cerebral AVMs is usually necessary with no more than 30% of the nidus obliterated in 1 session to prevent normal perfusion pressure breakthrough.
- •
Heparin is used when the venous outflow appears to be sluggish on the postembolization angiogram or if an important component of the venous outflow has been compromised.
Introduction
Arteriovenous malformations (AVMs) are relatively uncommon, highly complex vascular lesions that tend to occur in younger patients (20–40 years old). These lesions often become symptomatic with hemorrhage, seizures, headache, or focal neurologic deficits. Although treatment paradigms differ across institutions, the most compelling reason for the treatment of these lesions is to prevent hemorrhage. Existing data indicate that partial treatment of AVMs is not helpful and may increase the rate of future hemorrhage. The goal in treatment, therefore, is complete obliteration of the AVM. Given the complex morphology of AVMs and their frequent location in eloquent regions of the brain, no single treatment modality has proved to be effective. More so than for any other vascular lesion, a combined multidisciplinary approach is essential to formulate a safe and effective treatment strategy.
Introduction
Arteriovenous malformations (AVMs) are relatively uncommon, highly complex vascular lesions that tend to occur in younger patients (20–40 years old). These lesions often become symptomatic with hemorrhage, seizures, headache, or focal neurologic deficits. Although treatment paradigms differ across institutions, the most compelling reason for the treatment of these lesions is to prevent hemorrhage. Existing data indicate that partial treatment of AVMs is not helpful and may increase the rate of future hemorrhage. The goal in treatment, therefore, is complete obliteration of the AVM. Given the complex morphology of AVMs and their frequent location in eloquent regions of the brain, no single treatment modality has proved to be effective. More so than for any other vascular lesion, a combined multidisciplinary approach is essential to formulate a safe and effective treatment strategy.
Formulating a treatment strategy
The most critical step in the successful management of any AVM is formulation of a treatment plan based on an understanding of the natural history of the lesion and of the morbidity and mortality rates associated with various treatments.
Natural History
AVMs are usually identified after a hemorrhage ; therefore, the natural history of AVMs is not well described and is predominantly composed of retrospective analyses of selected populations (eg, those not undergoing surgery, patients with symptoms other than hemorrhage at presentation) yielding biased and relatively variable estimates of the rate of hemorrhage and its associated consequences. Given this bias, most series estimate that the annual risk of hemorrhage is 2% to 4% per year. In the year after a symptomatic hemorrhage, the rate of hemorrhage is believed to be higher, in the order of 6% to 18% per year, returning to the 2% to 4% baseline over time. AVM hemorrhages are not as severe as those associated with aneurysmal subarachnoid hemorrhage; after an AVM ruptures, the risk of death is 10% and the risk of major disability is 20% to 30%.
Morbidity and Mortality Rates Associated with Treatment
The risk of surgical intervention is directly related to the angioarchitecture and to the location of a particular AVM. This relationship is best represented by the Spetzler-Martin grading system, as modified by Spetzler and Ponce. In prospective studies, the Spetzler-Martin grade has been show to correlate reliably with surgical outcome.
Hamilton and Spetzler reported favorable operative morbidity and mortality rates of 1% and 3% for Grade I/II and Grade III AVMs, respectively. Morbidity and mortality rates in the immediate postoperative period associated with treating Grade IV and V AVMs may be as high as 31% and 50%, respectively. The morbidity and mortality rates decrease to 22% and 17%, respectively, at the time of the follow-up examination. Heros and colleagues reported a similar relationship between Spetzler-Martin grade and outcome. The aforementioned studies have provided the foundation for most management decisions related to treating AVMs.
Based on these studies, the risk of hemorrhage from Grade I and II AVMs outweighs the risk of surgical resection; therefore, these lesions are treated surgically, frequently without preoperative embolization, the risk of which can approach or even surpass the risk of surgery. Grade III AVMs represent a complex and at times challenging treatment conundrum. These lesions can be heterogeneous and associated with highly variable rates of morbidity and mortality. Lawton stratified these lesions into 3 additional angioarchitectural subcategories with low (2.9%), intermediate (7.1%), and high (14.8) risk of postsurgical death or new deficit. The treatment paradigm for these lesions involves preoperative embolization or radiosurgery followed by surgical resection.
The surgical resection of Grade IV and V AVMs is usually associated with a higher risk of morbidity and mortality than predicted from the natural history of these lesions. Han and colleagues analyzed outcomes in a series of 73 consecutive patients with Grade IV and V AVMs and recommended observation in most cases (55 of 73). The annual risk of hemorrhage in this group was 1% per year. Given the low rate of hemorrhage and the increased likelihood of hemorrhage from a partially treated lesion, the investigators recommend treatment of Grades IV and V AVMs only in patients with progressive neurologic deficits attributable to repeated hemorrhage or disabling symptoms, such as intractable seizures.
Indications for endovascular therapy for AVMs
The role of endovascular therapy in the management of cerebral AVMs depends on the overall treatment plan. The indications for embolization of AVMs from most to least common are as follows: (1) preoperative embolization as a precursor to complete curative surgical resection, (2) targeted therapy to obliterate a source of hemorrhage, (3) preradiosurgery as a precursor to radiation therapy, (4) curative embolization to attempt to cure small lesions, and (5) palliative embolization to relieve symptoms attributed to shunting.
Preoperative Embolization
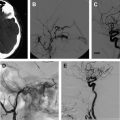
The embolization of an AVM is usually performed before curative surgical resection ( Fig. 1 ). The goal of embolization is to decrease the blood supply to the malformation, thereby decreasing the level of technical difficulty and associated morbidity of surgical resection. The endovascular interventionist must be aware of the surgical complication rate associated with the resection of the lesion being treated and must assure that the risks of the embolization do not exceed those of the surgical resection. For example, the risk of embolizing a Grade II AVM cannot exceed the risk of surgical treatment, given that these lesions can be safely resected with minimal complications. A successful embolization reduces the size of the AVM nidus, occludes deep feeding vessels that are difficult to access and control surgically, reduces intraoperative hemorrhage, and delineates surgical resection planes by leaving behind a cast of the lesion. Aided by the embolization, the goal of the vascular neurosurgeon is to achieve a complete, curative resection of the AVM.
Current data suggest that partial treatment of AVMs increases the risk of future hemorrhage. Han and colleagues observed a hemorrhage rate of 10.4% in patients with Grade IV and V AVMs after partial treatment, compared with a 1% risk in patients with observation. Miyamota and colleagues found an annual risk of hemorrhage of 14.6% in patients who underwent palliative treatment of cerebral AVMs. Wikholm and colleagues observed an increased rate of hemorrhage and death in patients undergoing partial treatment that resulted in less than 90% obliteration of the nidus.
The efficacy of embolization of AVMs using n -butyl cyanoacrylate (NBCA) has been demonstrated in several clinical studies. Jafar and colleagues demonstrated that the operative morbidity for large AVMs embolized preoperatively decreased to a level similar to that of small AVMs. DeMerritt and colleagues reported similar results with preoperative embolization of large AVMs improving postsurgical outcomes in comparison with a control group of smaller AVMs that were not embolized. More recently, Weber and colleagues reported their experience with Onyx for preoperative embolization and noted a mean nidus reduction of 84% after embolization in 47 patients. In their series of 28 patients, Natarajan and colleagues obliterated a mean of 74.1% of the preoperative volume.
Targeted Therapy
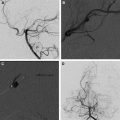
Partial obliteration of an AVM increases the likelihood of hemorrhage. However, in select patients with Grade IV and V AVMs, partial treatment targeted to eliminate an identified bleeding source may be undertaken. AVM-associated aneurysms are identified in 7% to 20% of cases and represent a significant risk factor for intracranial hemorrhage. These aneurysms may be flow related and are located on a feeding vessel or on vessels remote from the nidus. In some cases, an intranidal aneurysm or intranidal pseudoaneurysm composed of an organized hematoma that communicates with the intravascular space formed after AVM hemorrhage may be present. Both intranidal and extranidal aneurysms are risk factors for intracranial hemorrhage in patients with AVMs. The increased risk of hemorrhage in the setting of an extranidal aneurysm may be attributed to aneurysm rupture rather than to hemorrhage from the AVM nidus. When an unresectable AVM hemorrhages 1 or more times, endovascular exploration for a nidal aneurysm represents a reasonable strategy to embolize the aneurysm with a liquid embolic agent (nidal aneurysm) or coils (proximal flow-related aneurysm or remote aneurysm; Fig. 2 ).
Feeding vessel aneurysms can usually be identified by conventional angiography. Overlying vessels or other portions of the AVM nidus can obscure nidal aneurysms on conventional angiographic views. When aneurysms are difficult to visualize, superselective angiography, performed using high frame rates, can be used to identify and define the anatomy of lesions.
Preradiosurgery
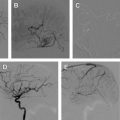
A detailed discussion of the role of radiosurgery for the treatment of AVMs is beyond the scope of this article, and the reader is referred to excellent reviews on this topic. The success of radiotherapy in treating AVMs is inversely proportional to the size of the nidus. The goal of embolization is to reduce the size of the AVM nidus to a range that is amenable to radiosurgical ablation ( Fig. 3 ), target nidal or feeding vessel aneurysms, and obliterate arteriovenous fistulae, which are usually refractory to treatment by radiosurgery. AVMs with nidal volume less than 10 mL (diameter <3 cm) can often be cured by radiosurgery, with rates of cure at 2 years estimated between 80% and 88%. In preradiosurgical embolization, the use of a permanent embolizate, such as NBCA (see later discussion), is essential to avoid recanalization of the portions of the embolized AVM not included in the radiation field.
The latency associated with radiosurgery to achieve a definitive effect on AVMs is 2 to 3 years. Of the available case series on combined endovascular/radiosurgical treatment of AVMs, most were conducted in the late 1980s and early 1990s, and many used particulate embolizates (eg, polyvinyl alcohol [PVA]).
The largest series, with 125 patients undergoing embolization (predominantly with NBCA) as a precursor to radiosurgery, achieved total occlusion in 11.2% of the AVMs after embolization alone; an additional 76% of lesions were reduced sufficiently to undergo radiotherapy. A 65% rate of total occlusion was observed after radiotherapy in patients undergoing combined treatment. More recently, Henkes and colleagues reported a series of 30 patients with high-grade AVMs undergoing combined embolization and radiotherapy. Their obliteration rate of 67% was less impressive. Andrade-Souza and colleagues reported nidus obliteration in 22 patients undergoing combined embolization and radiosurgery (47%) and in 33 patients (70%) undergoing radiosurgery alone. Their data suggest that preradiosurgery embolization may actually be detrimental to the effect of radiosurgery.
Several groups, however, have challenged this finding more recently. Back and colleagues reported that their patients who underwent embolization followed by unstaged Gamma Knife surgery had a follow-up rate of 53.5% (15 of 28) and an obliteration rate of 60.0% (9 of 15). Patients who underwent embolization followed by staged Gamma Knife surgery had a follow-up rate of 85.7% (6 of 7) and an obliteration rate of 66.7% (4 of 6). Blackburn and colleagues reported their experience with staged endovascular embolization followed by stereotactic radiosurgery for the treatment of large AVMs. Their AVM obliteration rate was 81% (13 of 16 cases). The newer data on combined endovascular/radiosurgical treatment of AVMs are more compelling and highlight this combined regimen as a viable option for the treatment of surgically challenging lesions.
Curative Therapy
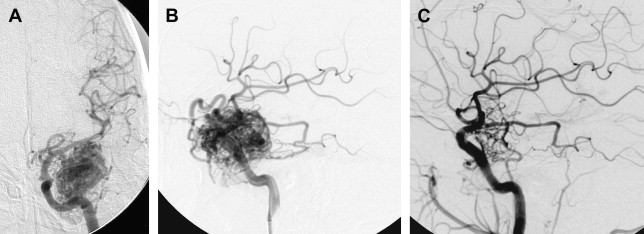
Occasionally, a small AVM with a limited number of feeding pedicles can be cured completely using endovascular embolization with a permanent agent (eg, NBCA or Onyx, not particles, Fig. 4 ). Most studies on the isolated use of embolization for treating AVMs predate the advent of Onyx (ev3, Inc, Irvine, CA). With the widespread application of this agent, obliteration rates may be expected to increase. Vinuela and colleagues reported a 9.7% cure rate for the embolization of small AVMs with few feeding pedicles. Gobin and colleagues reported a cure rate of 11.2% (14 patients) in a series of 125 patients undergoing preradiosurgical embolization. In this study, the chance of complete obliteration was inversely proportional to AVM volume and the number of feeding pedicles. Fournier and colleagues reported a cure rate of 14% with embolization alone. Wikholm and colleagues reported a complete obliteration rate of 13.3%, with success heavily dependent on the size of the AVM nidus: 71% for AVMs smaller than 4 mL and 15% for AVMs between 4 and 8 mL. Yu and colleagues reported a 22% cure rate in 27 patients treated with cyanoacrylate embolization alone. These investigators found that the angiographic obliteration of the AVM was durable at 17 to 32 months with no recurrences or complications.
Katsaridis and colleagues reviewed their experience with 101 patients treated with Onyx and identified total occlusion of AVMs in 28 of 101 (27.7%) and near total occlusion in 18 of 101 (17.8%). Their results are primary, as only 52 patients in their series had been treated completely; the remaining 49 required further embolization. They concluded that, with the introduction of Onyx, more AVMs would be amenable to complete curative embolization.
Similarly Maimon and colleagues obtained complete obliteration by using embolization in 16 patients, resulting in a 55% cure rate in patients who concluded treatments (16 of 29). Abud and colleagues used double arterial catheterization with simultaneous injection of Onyx in 17 patients and obtained curative embolization in 16 patients (94.1%). They advocated the use of this technique because it allows controlled hemodynamic filling of the AVM nidus.
Valavanis and Christoforidis reported substantially higher cure rates (40%) in a consecutive series of 387 patients. These investigators identified the presence of direct, dominant feeding arteries, a monocompartmental nidus, and a dominant fistulous component of the nidus, without perinidal angiogenesis as the key characteristics predictive of endovascular cure. These investigators did not find size or number of feeding pedicles to be important determinants of the potential for endovascular obliteration. Others, however, have clearly documented both size and feeding number of pedicles as important determinants of the likelihood for cure.
Palliative Therapy
Although the point is controversial, some investigators theorize that large AVMs cause progressive neurologic deficits, intellectual deterioration, or persistent headaches as a result of a vascular steal phenomenon, via shunting. There is currently minimal evidence to support partial embolization in cases of suspected cerebrovascular steal. Despite reports of increased likelihood of hemorrhage associated with partially treated AVMs, some investigators have advocated palliative, partial embolization to reduce the severity of arteriovenous shunting and to improve perfusion pressure in functional brain parenchyma.
No large clinical series support this strategy, but several case reports have described success in small numbers of patients. Fox and colleagues reported that limb weakness improved in 3 patients after subtotal embolization of large AVMs located near the motor cortex. They attributed the improvement to a reduction in cerebrovascular steal. Rosenkranz and colleagues reported 2 patients who underwent palliative embolization with resolution of the effects of shunting and intracranial hypertension secondary to inoperable AVMs. Simon and colleagues report a single case of a patient whose symptoms of trigeminal neuralgia resolved after palliative embolization of a cerebellopontine angle AVM. The patient had a recurrence after 17 months and underwent another bout of palliative embolization. From a practical perspective, when such a strategy is undertaken, it is imperative to proceed cautiously and to avoid minimizing venous outflow.
Embolizates
Liquid Embolics
Liquid embolic agents are the most widely used and most effective for AVM embolization. The cyanoacrylate polymers (eg, NBCA) most commonly used of the liquid agents. The dimethyl sulfoxide (DMSO) solvent-based system ethylene-vinyl alcohol (EVOH) copolymer (Onyx) is a liquid nonadhesive embolic agent that became available in the United States in late 2005. In terms of its safety and efficacy, Onyx has recently been shown to be equivalent to NBCA as a preoperative embolic agent for reducing brain AVMs. Ethanol (EtOH), an agent used effectively in treating peripheral AVMs, has been used successfully to treat cerebral AVMs.
Cyanoacrylate
Cyanoacrylates are liquid adhesive polymeric agents with several important advantages: (1) the potential for deep penetration into the AVM nidus; (2) permanent embolization with durable occlusion of the embolized vessel or pedicle; (3) the ability to be delivered through small, flexible, flow-directed catheters that can be manipulated safely and atraumatically into the most distal locations within the cerebrovasculature ( Fig. 5 ); and (4) the ability to be delivered into the pedicle easily and quickly, with infusions usually requiring less than 1 minute. Several different cyanoacrylates have been used. The first agent available was iso-butyl-2-cyanoacrylate, but its use has now been discontinued after studies demonstrated that it possessed carcinogenic potential in animals.
NBCA is now the cyanoacrylate of choice for AVM embolization. Cyanoacrylates are introduced as liquid monomers that polymerize to form a stable solid when they contact a solution containing anions, such as the hydroxyl groups in blood. The rate of polymerization and the rate of injection determine how far the agent travels within the cerebral vasculature before solidifying. The NBCA itself is radiolucent and must be mixed with a radiopaque agent, typically ethiodized oil (eg, Lipiodol, Ethiodol). For most applications we use a 1.5:1 to 3:1 (oil/NBCA) mixture. In addition to imparting radiopacity to the NBCA, the oil acts as a retardant, slowing the rate of polymerization and allowing the NBCA to travel further in the vessel before it solidifies. Glacial acetic acid can also be added to the mixture in small quantities to retard the rate of further polymerization.
After solidifying, the cyanoacrylates (if a sufficient volume has been injected) immediately occlude the embolized pedicle. The intense inflammatory reaction that follows leads to fibrous ingrowth, which, in turn, produces a durable occlusion. Although recanalization can occur, it is rare after adequate embolization. Some disadvantages of the liquid adhesives include the high level of expertise required to control the injection safely to achieve adequate nidal penetration without allowing the agent to extend into the vein and the risk of NBCA adhering to the catheter, making it traumatic or impossible to withdraw the catheter.
EVOH Copolymer-DMSO Solvent
EVOH is a radiolucent polymeric agent that, in some respects, is similar to NBCA. This agent was first applied to the treatment of AVMs in the early 1990s and has been commercially available in the United States as Onyx since late 2005. The most significant advantage of the EVOH copolymer is that it is nonadhesive, reducing the possibility of the catheter adhering to the injected polymer. The operator therefore has a greater degree of flexibility in terms of the volume and rate of the injection. Periodically, the operator may temporarily halt an EVOH-DMSO infusion to perform control angiography and to assess the status of the AVM nidus and draining veins before continuing the infusion.
Initial studies showed that the DMSO component of the mixture induced vasospasm and angionecrosis. Jahan and colleagues identified 1 complication (proximal reflux) related to distal vasospasm that developed during an injection. This same group also reported histopathologic evidence of angionecrosis in 2 AVM specimens resected 24 hours after embolization. Subsequent investigations indicated that the deleterious effects of DMSO could be eliminated by limiting its volume and rate of introduction. Given the radiolucency of the EVOH-DMSO, tantalum powder must be mixed with the agent to provide radiopacity. Failure to mix the EVOH-DMSO-tantalum preparation constantly results in sedimentation of the tantalum from the mixture with subsequent variable opacification. The result is suboptimal visualization of the embolizate during its injection. Although long-term data are lacking, EVOH is, like NBCA, for all practical purposes, a permanent agent.
EtOH
EtOH is a sclerosant, functioning to dehydrate and denude the endothelium, creating fractures within the vessel wall that extend to the level of the internal elastic lamina. These changes result in acute thrombosis of the vessel. EtOH causes significant brain edema, necessitating treatment with high doses of steroids immediately before the procedure and for 2 weeks after. In some cases, brain edema and increases in intracranial pressure necessitate mannitol therapy. In high doses, EtOH can induce pulmonary precapillary vasospasm, which can lead to cardiopulmonary collapse. This effect has been reported in humans after the embolization of peripheral AVMs with EtOH.
Based on the success associated with the use of EtOH in treating peripheral vascular malformations, Yakes and colleagues advocated the use of undiluted absolute ethyl alcohol (98% dehydrated alcohol injection) for the embolization of AVMs involving the central nervous system. In their initial series of 17 patients, they obtained total occlusion in 7 patients with EtOH alone; 3 additional patients were cured after surgery and another 1 after radiotherapy. Despite this impressive cure rate, there was a significant complication rate. Two patients with partially treated lesions died and 8 patients had treatment-related complications.
Others have reported intraprocedural complications associated with the use of EtOH. Unnikrishnan and colleagues used EtOH to embolize a left parietooccipital AVM under general anesthesia. Despite general anesthesia, during injection of absolute EtOH into the AVM nidus, the patient developed hypertension and tachycardia coincident with a profound and sustained reduction of bispectral index values. This patient did not have a hemorrhage, but the hypertension and tachycardia are certainly worrisome byproducts of EtOH use. No other similar case series describing the application of EtOH have been reported to date. Given the risks associated with the use of EtOH, the high level of procedure-related complications, and the relatively widespread experience and comfort level with the cyanoacrylates, there has been a general reluctance among most endovascular interventionalists to use EtOH to embolize brain AVMs.
Particles
Many different particulate embolizates, including silk sutures, microfibrillar collagen material, PVA, and embolization microspheres, have been used to embolize AVMs.
PVA/Embospheres
Technically, embolization with particulate agents is fundamentally different from embolization with NBCA. To perform particulate embolization, a microcatheter with an internal diameter large enough to accept the particulate agent without clumping and clogging must be used. These catheters have a higher profile and are considerably less flexible than the smaller internal diameter flow-directed catheters. Given the structural limitations of these catheters, an over-the-wire technique must be used to negotiate the microcatheter into the region of the AVM nidus. These technical factors make superselective catheterization of pedicles feeding the nidus more labor intensive and more hazardous with a greater potential for vascular perforation.
After a pedicle has been catheterized, the size of the particles chosen is based on the superselective angiogram. If superselective angiography shows large shunts, they must initially be occluded with coils to avoid direct arteriovenous shunting of the particles into the pulmonary circulation. Next, the particles must be injected through the catheter gradually to occlude the vessels supplying the nidus with intermittent control angiography to assess flow. Unfortunately, the vessels coursing to the nidus are frequently of various calibers with differing degrees of shunting, making the selection of optimally sized particles challenging. Furthermore, multiple injections are typically required to occlude the pedicle over minutes rather than over seconds, as for NBCA.
In addition to the increased procedural time, there is the theoretic concern of temporarily pressurizing the nidus, as the higher flow, lower pressure, fistulous components become preferentially occluded. Theoretically, this situation increases the potential for intraprocedural hemorrhage. Particulate agents have also been reported to be more prone to recanalization than cyanoacrylates. Sorimachi and colleagues reported a 43% rate of nidal recanalization after particulate embolization with PVA. Mathis and colleagues reported a 12% recanalization rate for AVMs embolized with PVA in preparation for radiosurgery when portions of the AVM were not included in the radiation field.
Given the availability of more permanent embolizates, particulate agents are relatively contraindicated for the embolization of AVMs. Wallace and colleagues reported a retrospective comparison of outcomes for 65 patients with AVMs embolized with either PVA or NBCA. The lowest complication rate was associated with NBCA embolization, which they attributed to a lower surgical complication rate. In a larger prospectively randomized trial comparing NBCA and PVA, the equivalency of the 2 agents was demonstrated with respect to the degree of nidal reduction, the number of vessels embolized, surgical resection times, transfusions, fluid replacement, and Glasgow Outcome Scale scores. A significant difference was identified only with respect to the rate of postsurgical hematoma, which was greater after PVA (8 of 45) than after NCBA (2 of 42).
Coils
Coils, both detachable (eg, Guglielmi detachable coils) and injectable (Berenstein liquid coils), are useful for the occlusion of arteriovenous fistulae within the AVM nidus. The introduction of coils into the friable arterial feeders of an AVM, however, represents a risk of perforation. Detachable coils are most useful for the initial embolization of large fistulae. Depending on the size of the artery to be embolized, we usually select a complex three-dimensional geometry or a fibered 0.457 mm (0.018-in) detachable coil for the first coil. The coil is selected based on the size of the feeding artery as estimated on superselective angiography or guiding catheter angiography if the volume of shunting precludes complete opacification of the vessel after a microcatheter injection. We oversize the coil by 1 to 2 mm and choose the longest available coil that satisfies the diameter requirements. This coil can be manipulated within the feeding artery to achieve optimal positioning.
Visualization of the nondetached coil under fluoroscopy can provide some indication of its stability within the artery. After detachment of the first coil, a second soft coil of a diameter similar to the artery in the greatest length available is immediately introduced. After several coils have been introduced and a stable basket has been created, 1 or more liquid coils (0.254-mm [0.010-in] Berenstein liquid coils; Boston Scientific, Natick, MA) may be deployed. After the coil pack has adequately slowed the flow through the fistula, the pedicle may be occluded definitively with an injection of NBCA. Frequently, it is useful to induce hypotension (systolic blood pressure <90 mm Hg) for the NBCA injection to reduce the risk of NBCA passing through the fistula and into the venous system.
When detachable coils are used, the over-the-wire manipulation of a microcatheter with 2 distal markers into the pedicle is necessary, introducing the potential for vascular perforation. For this reason, it is useful to have an appropriate ethiodized oil/NBCA (2:1) mixture prepared for use before cannulization of the pedicle to be coiled.
If the coils are sized improperly, there is the potential for embolization through the fistula and into the venous system. If the arterial pedicle supplying the fistula is small enough, embolization with injectable or small 0.254 mm (0.010-in) pushable coils can be performed primarily. Liquid coils may be introduced through the smaller internal diameter, flexible, flow-directed microcatheters, eliminating the need for an exclusively over-the-wire catheterization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree