Esophagus
Wolfgang A. Weber
Richard L. Wahl
Esophageal carcinoma is considered the eighth most common cancer worldwide with nearly 400,000 new cases diagnosed annually. However, incidence rates of esophageal cancer vary greatly from one region to another. Incidence rates are highest in Asia and southern and eastern Africa. In these areas the incidence of esophageal cancer has been reported to be as high as 100 per 100,000 (1). In the United States, esophageal carcinoma is a relatively uncommon cancer, with an estimated incidence rate of 5.4 per 100,000 corresponding to 16,470 new cases in 2006 (12,970 men and 3,500 women) (2). The cancer is extremely lethal and has an estimated death rate of 14,280 patients per year in the United States, with 11,250 men and 3,030 women dying. This high mortality rate is consistent with a very low 5-year survival after diagnosis, which is only about 16% for all stages of the disease. Although the 5-year survival is still poor, it has improved slightly, but significantly (P<.05) from 5% in 1975 to 1977 to 16% in 1996 to 2003, presumably due to improved diagnosis and treatment (2).
Most esophageal cancers diagnosed worldwide are of squamous cell histology. However, in the Western world, there has been a substantial increase in the incidence and prevalence of adenocarcinoma of the esophagus, particularly those arising at the esophagogastric junction (3). In the United States, the incidence of adenocarcinoma of the esophagus has increased about fourfold between 1973 to 1982 and 1993 to 2002 (3). Most, if not all, adenocarcinomas of the distal esophagus arise from areas with specialized intestinal metaplasia, which develop as a consequence of chronic gastroesophageal (GE) reflux. In some patients, it can be difficult to determine if tumors at the GE junction arose from the esophagus or the stomach itself. A substantial focus on the prevention of GE reflux disease, including prevention and treatment of Barrett’s esophagus, is an ongoing attempt to reduce the increase of adenocarcinoma of the esophagus in the Western world. When gastric carcinomas occur in the fundus, they can be difficult or impossible to distinguish from esophageal adenocarcinomas.
The most common sites of metastatic disease are the locoregional lymph nodes immediately adjoining the esophagus and the upper abdominal lymph nodes. Tumors in the lower esophagus and GE junction may metastasize to mesenteric nodes. Tumors of the upper esophagus commonly metastasize to the cervical nodes, as well as the liver, lungs, and other organs including bone.
Decisions concerning primary therapy of esophageal cancer are based on knowledge of the tumor stage. Staging for esophageal carcinoma is commonly tabulated using the American Joint Committee on Cancers Tumor, Node, Metastasis (TNM) system (Tables 8.13.1 and 8.13.2). Depending on tumor stage, current potentially curative treatment options for esophageal and gastric cancer range from endoscopic mucosal resection to preoperative chemoradiotherapy followed by esophagectomy (4). Most of these therapeutic approaches are associated with substantial morbidity and mortality as well as a long-term compromise in the quality of life. Accurate pretherapeutic staging by imaging techniques is therefore crucial in order to select the appropriate form of therapy. Generally the first step in this process is to distinguish between patients with locoregional and systemic disease. For patients presenting with hematogenous metastases or distant lymph node metastases (M1), no curative treatment is available and local tumor therapy is only applied for palliation of symptoms.
In patients with locoregional disease, assessment of local tumor infiltration (T stage) and regional lymph node involvement (N stage) is necessary in order to decide whether a complete tumor resection (R0) is feasible (5). Local tumor infiltration (T stage) is also frequently used as a criterion to use multimodality therapy instead of primary surgery. Although patients with T1 and T2 tumors are generally treated by primary resection and lymph node dissection, patients with T3 and T4 tumors are frequently offered preoperative chemo- or chemoradiotherapy in order to improve the rate of curative resections and potentially overall survival (6). When the primary tumor is limited to the mucosa or submucosa (T1), limited surgical resections may provide the same chance for cure as standard surgical procedures. As the frequency of lymph node metastases is very low in tumors limited to the mucosa, patients with these early cancers may even be cured by endoscopic mucosal resection (7).
Survival is dependent on stage and is much better for localized than locoregional or disseminated esophageal cancers. For patients with stage I esophageal cancer 5-year survival rates of 80% have been reported after surgical resection (8,9). Various methods have been used to stage esophageal cancer, including endoscopic ultrasound (EUS), computed tomography (CT), and more recently positron emission tomography (PET) imaging, particularly using fluorine-18 (18F) fluorodeoxyglucose (FDG).
Table 8.13.1 Tumor, Node, Metastasis Staging System for Esophageal Cancer | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
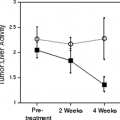
Imaging techniques are also used to evaluate tumor response to chemo- and chemoradiotherapy. Tumor response to preoperative therapy is a very strong prognostic factor in esophageal cancer (8). Multiple studies have demonstrated that patients with a substantial reduction in the number of viable tumor cells are characterized by a relatively favorable prognosis (5-year survival greater than 50%), even if they presented with locally advanced disease at diagnosis (8,10). Noninvasive assessment of tumor response thus provides important prognostic information and can be used to guide further patient management.
Table 8.13.2 American Joint Committee on Cancer Stage Groupings | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Primary Tumor Detection
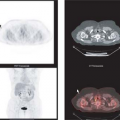
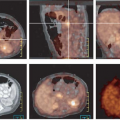
Nearly all localized primary esophageal carcinomas are detected first by clinical symptoms of dysphagia or in the context of endoscopy performed as part of an evaluation of the upper gastrointestinal (GI) tract for GE reflux—or in sequential management of patients with Barrett’s esophagus. PET has not been used as a primary screening method for esophageal carcinoma. The precursor lesion to many cases of adenocarcinoma of the esophagus is Barrett’s esophagus. It usually has a low total volume that would not be expected to be detected using whole-body PET imaging devices, although on rare occasions it can be (11). Similarly, some inflammations of the distal esophagus can have mildly increased FDG uptake (11). Several studies have shown that the vast majority of primary esophageal cancers that are first diagnosed by other methods are detectable by [18F]-FDG PET, with sensitivities in the 90% to 100% range for T2 to T4 tumors (12,13,14,15,16). When PET results have been reported to be falsely negative in esophageal cancers, this was usually due to small tumor volume, such as in stage T1 primary lesions. Furthermore, some adenocarcinomas of the gastric cardia demonstrate only low FDG uptake and can be falsely negative on FDG PET even at advanced tumor stages. This is likely related to their growth pattern and mucin production (17).
Diagnostic challenges in esophageal cancer include determining whether there is abnormal or physiologic uptake at the GE junction. There may be some uptake in this location normally, so detecting small esophageal cancers can be problematic as they can be lost in the normal spectrum of mild FDG uptake in the distal esophagus. For these reasons, it is probable that early low-volume esophageal cancer can be much more easily detected by direct visualization using an endoscope or by careful barium studies than by PET.
Locoregional Nodal Detection
The detection of nodal metastases with PET is dependent on the volume of tumor in the metastasis, the intensity of tracer uptake in the lesion, the background tracer activity (as well as the injected dose of radiotracer and the performance and resolution of the scanner), and the interpretation criteria. CT detects nodal metastases based on their size, and what constitutes a “positive or negative” node by CT differs with different observers. The larger the node, the more likely it is to represent a node involved by cancer, although the optimal cutoff size for “positive or negative” nodes is not clear in esophageal cancer.
Both PET and CT can fail to detect small metastases of esophageal cancer to locoregional lymph nodes. Lesions smaller than 5 mm are not usually detected on PET with FDG, which is consistent with other detection challenges encountered with current FDG PET technology. To achieve high sensitivity with CT, small lymph nodes must be called positive. For example, 5-mm and larger nodes may be called abnormal in some CT studies, whereas others may choose a 10-mm cutoff. The smaller the cutoff for node size, the more likely cancer will be detected, but at the price of a lower specificity. This results in a considerable range in the sensitivity and specificity of PET for assessing tumor involvement in regional lymph nodes and an even greater range in accuracy of CT (Table 8.13.3).
In general PET is not exceptionally sensitive, but it has a high specificity for the detection of locoregional lymph node metastases (typical sensitivities of 30% to 80%, but with specificities of 80% to 90%). A meta-analysis published in 2004 evaluating data from 421 patients reported a pooled sensitivity and specificity of FDG PET of 51% and 84%, respectively. Table 8.13.3 provides an overview of the largest studies used in the meta-analysis as well as of more recently published studies (18). By contrast, reported CT sensitivity/specificity pairs range from (31% to 86% to 87% to 14%), meaning CT can be either very insensitive or reasonably specific (although not as specific as PET) or very sensitive and extremely nonspecific. When directly compared, in several studies PET was an equivalent or more accurate method for nodal staging than CT (Table 8.13.3).
Table 8.13.3 Diagnostic Accuracy of Fluorodeoxyglucose- PET and CT for Detection of Locoregional Lymph Node Metastases (N stage) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EUS has been reported to be more sensitive (70% to 80%) than either PET or CT for staging regional nodes and it is reasonably specific (70% to 80%) (15,19,20). EUS is also valuable for assessing tumor size and depth of invasion. According to a systematic review including a total of 2,508 patients, the diagnostic accuracy of EUS for differentiation of stages T1 and T2 from stages T3 and T4 was 91% (21). There are no data showing that PET or CT is accurate in evaluating the primary tumor stage of esophageal cancers. Neither can resolve the individual layers of the esophageal wall that form the basis of the T-staging system. A disadvantage of EUS is its operator dependency. Furthermore, EUS may be technically impossible if the tumor causes a stenosis that cannot be passed by the endoscope (21). There is some interest in using minimally invasive surgical techniques for staging esophageal cancer. Specifically, sentinel node dissection is currently being evaluated as a minimally invasive technique to improve lymph node staging (22).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree