10 EUS-Guided Biopsy—Indications, Problems, Pitfalls, Troubleshooting, and Clinical Impact Endoscopic ultrasound-guided needle biopsies (EUS-guided fine-needle aspiration, EUS-FNA; EUS-guided Tru-Cut biopsy, EUS-TCB) are reliable, safe, and effective techniques for obtaining samples for cytological or histological examinations, either as a primary procedure or in cases in which other biopsy techniques have failed. EUS-FNA and EUS-TCB have become cornerstone techniques in visceral medicine, pulmonary medicine, and oncology. EUS-guided needle biopsy (EUS-NB) has considerably increased the diagnostic potential of endoscopic ultrasound (EUS). Between May 1, 2008, and April 3, 2009, 58 of 185 (31.3%) original publications on EUS reported on EUS-NB.1 The initial reports on EUS-FNA were published in gastroenterology journals in 1992.2,3 EUS-NB has also in the meantime become a topic in the cytopathology literature, following an initial report in 1997.4 Cytopathological diagnoses based on EUS-NB may have a major impact on therapeutic decision-making and on the individual prognoses for patients—avoiding major surgery, dispensing with invasive diagnostic interventions such as thoracoscopy, mediastinoscopy, and laparoscopy, and determining whether neoadjuvant treatment or nonsurgical therapy is needed. This is the reason why the endosonographer and cytopathologist share a common responsibility for the quality with which biopsy material is acquired and processed, as well as for the cytological and histological diagnosis. This chapter discusses the problems and pitfalls of EUS-NB from the clinical and cytopathological points of view and considers reasonable solutions for problems that arise.
Nonresectable tumors |
Cytological/histological diagnosis before initiation of chemotherapy |
Proof of nonresectability (liver metastasis, mediastinal lymph-node metastasis, pleural and peritoneal carcinosis) |
Resectable tumors |
Suspicion of solid tumors other than ductal adenocarcinoma (e.g., neuroendocrine tumor, malignant lymphoma, pancreatic metastases) |
Differentiation of cystic pancreatic lesions |
Suspicion of ductal adenocarcinoma only in cases in which the surgical decision depends on cytological/histological diagnosis |
Unclear findings |
Cytological/histological proof of a benign diagnosis in case of a low pretest probability for a malignant tumor (e.g., focal pancreatitis, autoimmune pancreatitis) |
Indications and Contraindications
EUS-NB is indicated for cytopathological diagnosis of mass lesions in the gastrointestinal tract and adjacent to it, and of lymph nodes in its vicinity, if sampling using less invasive methods (e.g., forceps biopsy) is not possible or has failed. EUS-NB can therefore be of considerable value in the primary diagnosis and staging of malignant diseases, as well as for differentiating benign diseases. For EUS-NB to be justified, there must be potential clinical implications of the resulting cytopathological diagnosis—for example, a change in diagnostic pathways, avoidance of invasive or surgical treatment, or a decision in favor of specific treatments (Tables 10.1, 10.2, 10.3, 10.4).5–8 EUS-NB is contra-indicated in all circumstances in which the risks of the procedure outweigh the expected benefits of the diagnostic information it can provide (Table 10.5).
Primary diagnosis |
Method of first choice in hypoechoic subepithelial tumors: gastrointestinal stromal tumor (GIST), schwannoma, leiomyoma, neuroendocrine tumor, granular cell tumor, etc. |
After failure, or if other biopsy methods are contraindicated, in diffuse infiltrating gastric cancer (linitis plastica), cholangiocarcinoma, hepatocellular carcinoma |
Staging |
Esophageal cancer: celiac lymph-node metastasis, liver metastasis, peritoneal carcinosis |
Biliaryand gastrointestinal cancer: liver metastasis, peritoneal and pleural carcinosis, adrenal metastasis, mediastinal lymph-node metastases |
Follow-up |
Diagnosis of extraluminal recurrence after surgery for rectal cancer or other gastrointestinal malignancies |
Primary diagnosis |
Suspicion of lung cancer: after failure of bronchoscopic biopsy (central, periesophageal tumors; lymph-node metastases; metastases in the left adrenal gland or the liver) |
Staging |
Mediastinal staging: proof of N2 or N3 metastasis (NSCLC) orany mediastinal lymph-node metastasis (SCLC); proof of pleural carcinosis |
Infradiaphragmatic staging: proof of distant metastasis (adrenal gland, liver, infradiaphragmatic lymph node) |
Follow-up |
Diagnosis of recurrence after curative surgery for lung cancer |
Diagnosis of ambiguous and benign mass lesions |
Ambiguous mediastinal lymphadenopathy or solid mass lesions |
Suspicion of sarcoidosis or tuberculosis |
NSCLC, non-small cell cancer; SCLC, small cell lung cancer.
Primary diagnosis |
Retroperitoneal tumors (adrenal glands after exclusion of pheochromocytoma; mesenchymal tumors) |
Suspicion of malignant lymphoma (mediastinal and infradiaphragmatic lymph nodes, splenic lesions) |
Suspicion of inflammatory mass lesions (mediastinum, retroperitoneum, gastrointestinal wall) |
Staging |
Various cancers: liver metastasis, adrenal metastasis, splenic metastasis, mediastinal or infradiaphragmatic lymph-node metastases, peritoneal and pleural carcinosis |
Follow-up |
Diagnosis of recurrence after curative treatment for various malignancies |
No informed consent |
Lack of cooperation or insufficient sedation |
Anticoagulation treatment or coagulopathy (international normalized ratio INR > 1.5; platelet count <50000; heparin in therapeutic doses) |
Inhibition of platelet aggregation by clopidogrel9 |
Failure of ultrasound needle control |
EUS-NB of the liver, pancreatic head, or ampulla in cases of insufficient drainage of obstructed bile ducts |
Results of EUS-NB are unlikely to have a significant clinical impact |
a A relative contraindication; benefit has to be weighed against risk very carefully.
b Peri-interventional antibiotic treatment is mandatory. EUS-NB, EUS-guided needle biopsy.
c Transaortic EUS-FNA of mediastinal lymph nodes and tumors,10,11 of the pericardium and a left a trial mass,12 and of intravascular tumors13 without any major complications has been reported.
Before EUS-guided biopsy is carried out, all of the endoscopic and imaging studies available—such as transabdominal ultrasound (TUS), computed tomography (CT), and magnetic resonance imaging (MRI)—should be reviewed in order to generate a “road map” of the procedure. The endoscopy suite and team should be adequately prepared. The indication for EUS-NB has to be verified, and contraindications and risk factors must be checked. We would suggest that a complete EUS examination should first be conducted, looking specifically for findings that would probably represent the most advanced stage of a malignant gastrointestinal process—for example, a celiac lymph node in a patient with suspected esophageal cancer, or a liver lesion, ascites, or a mediastinal lymph node in a patient with a suspected pancreatic mass lesion. If malignant disease has been confirmed or is suspected, the first EUS-NB procedure should always target the lesion that would prove to represent the most advanced stage in case of positive cytological or histological findings (Fig. 10.1)7.
Fig. 10.1 An algorithm for EUS-NB in oncological staging. Sampling should start in lesions in which positive findings would confirm the presence of distant metastases, then proceed to possible lymphnode metastases, and conclude with the suspected primary tumor, if needed (inverse TNM system).
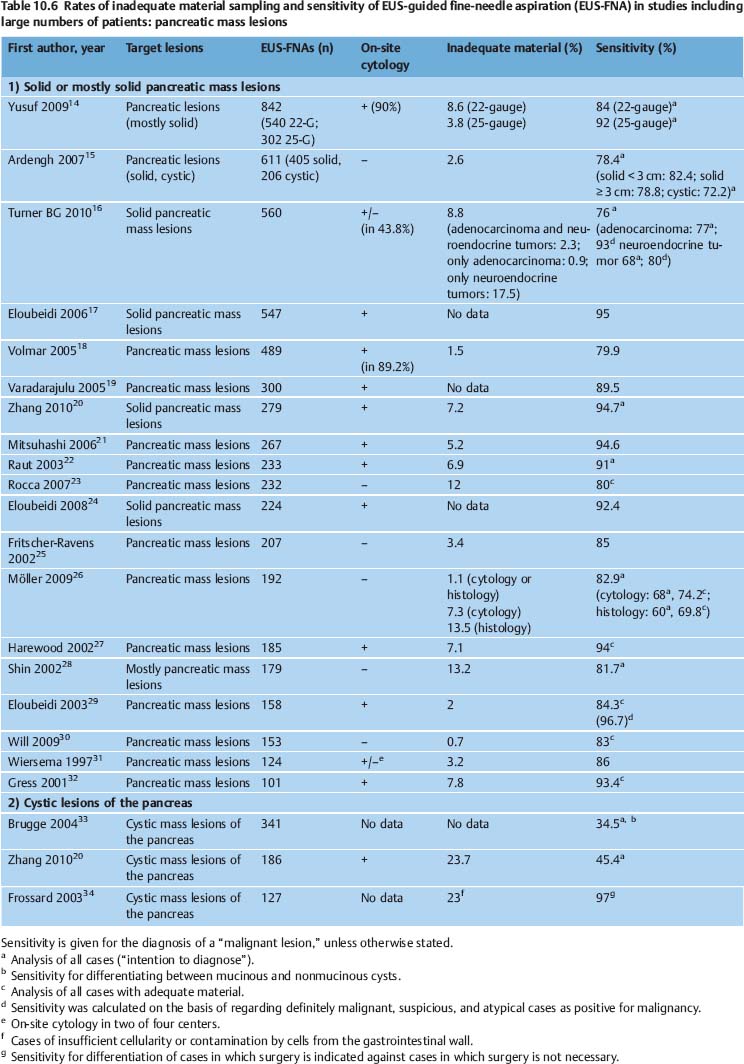
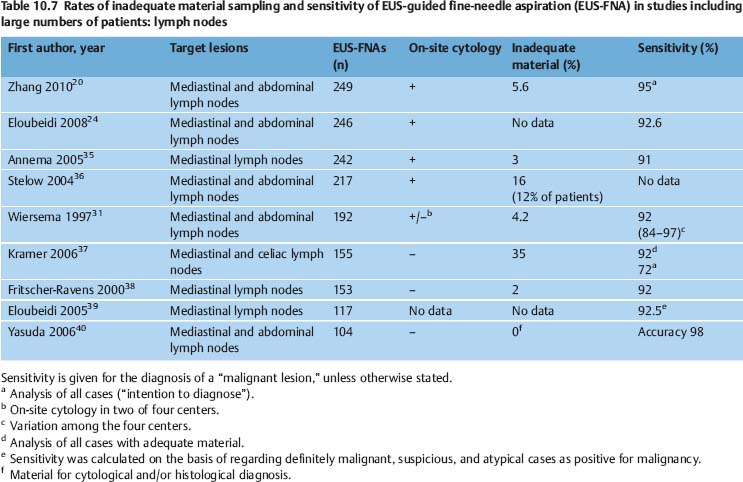
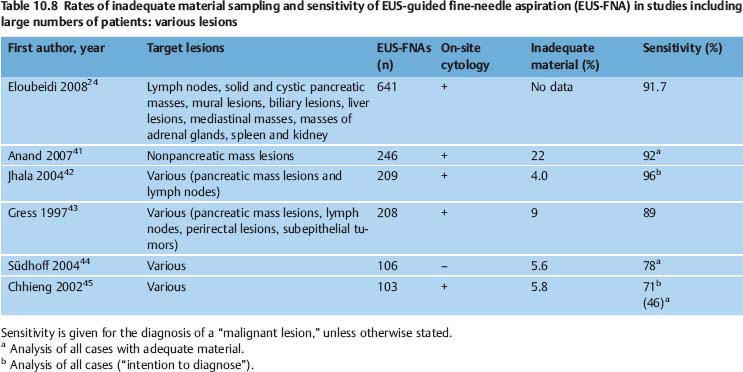
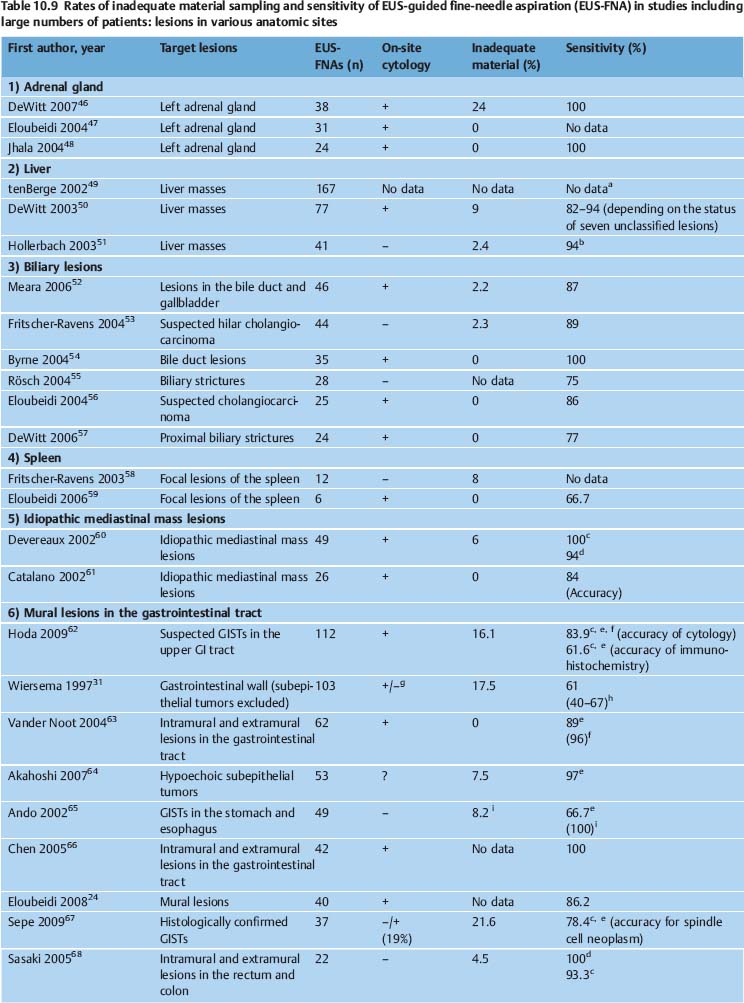
The diagnostic yield of EUS-NB varies depending on the nature of the target lesion, the skill and experience of the endoscopist and cytopathologist, and on numerous technical and procedural variables. It can potentially be improved with additional techniques such as real-time elastography and contrast-enhanced EUS. In various studies, EUS-FNA of solid pancreatic masses provided adequate material for cytological examination in 86.8–98.5% of cases, and for histological assessment in 68.9–89.0% of cases. The diagnostic yield of EUS-FNA was 65%-100% for lymph nodes, 91–100% for solid liver lesions, 97.7–100.0% for biliary structures, and 82.0–91.8% for subepithelial tumors of the gastrointestinal tract (Tables 10.6, 10.7, 10.8, 10.9).
It is difficult to compare sensitivity and specificity data between different studies. In some studies, inadequate biopsies are excluded from the statistical analysis, while others treat these as false-negatives. In statistical analyses, some authors treat “suspicious for malignancy” and “atypical” cytological findings as negative findings (true or false negatives), while others regard suspicious and sometimes even atypical findings as true or false positives. Rating suspicious findings as true positives is based on observations from EUS-FNA studies of solid pancreatic mass lesions, in which 82–100% of cases of suspicious cytology proved to be malignant neoplasms during the follow-up.18,29,73–75 Different gold standards have also been used for statistical evaluation of EUS-NB studies. Most studies have used a combination of surgical pathology, other histological findings, radiological studies, and clinical follow-up to confirm a malignant diagnosis. Only a few studies have correlated the findings of EUS-NB with surgical pathology in all or almost all cases.65,76–80
Another problem causing difficulties in comparing the results of EUS-NB studies is the absence of a consensus definition of “inadequate,” “insufficient,” and “nondiagnostic” biopsy findings. Mitsuhashi et al. assessed smears as satisfactory if unequivocally malignant cells were identified. For negative cases, at least 5–10 groups of ≥ 10 epithelial cells or pancreatic acini had to be present on each slide. When only blood or normal gastrointestinal epithelial cells were seen, the specimen was regarded inadequate.21 Noh et al. defined smears with two or three clusters of benign or malignant pancreatic glandular cells as adequate. Fordiagnostic smears from EUS-FNA of lymph nodes, the same authors postulated the presence of abundant lymphocytes and tingible body macrophages.81 In studies on EUS-FNA including large numbers of patients, the reported rates of inadequate smears vary very widely (0–35%) depending on the definition of adequacy and the type of target lesion (Tables 10.6, 10.7, 10.8, 10.9).
There are few published data on the ability of EUS-FNA to provide adequate material for cytological or histological assessment. Combining cytological and histological analyses of samples obtained by EUS-FNA may improve diagnostic accuracy slightly (Table 10.10). The effectiveness of EUC-TCB in providing core biopsies of solid pancreatic masses, lymph nodes, and various other solid lesions varies between 50% and 99%(Table 10.11).
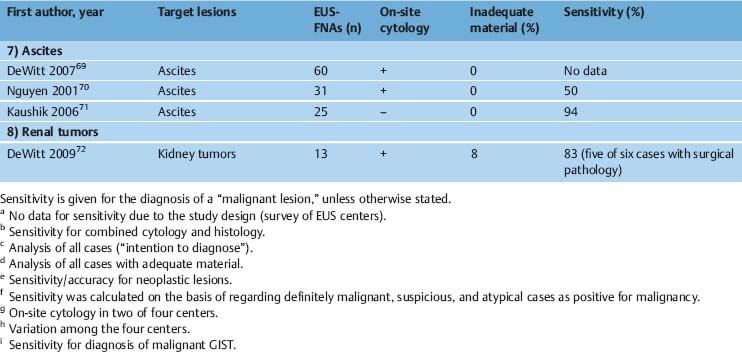
Factors | Relevance | Problem |
1) Target organ, target tissue |
|
|
Anatomic site | + | Problems in obtaining diagnostic material: pancreatic head (especially uncinate process) and neck, gastrointestinal wall lesions; interposition of vessels |
Biological tissue characteristics | + | Problems in obtaining diagnostic material: benign lesions, well-differentiated ductal pancreatic adenocarcinoma |
Type of neoplasia | ++ | Problems in obtaining diagnostic material: subepithelial gastrointestinal tumors, pancreatic cancer, cystic lesions |
Vascularization of target lesion | + | Bloody contamination of aspirates |
Size of lesion | + | Problems in obtaining diagnostic material: very small lesions, very large and necrotic mass lesions |
2) Methodological aspects |
|
|
Type and diameter of needle | + | Influence on: diagnostic yield (cytology, histology), technical feasibility |
Aspiration | + | Influence on: cellularity, yield of histological material, bloody contamination |
Number of needle passes | ++ | Dependent on target organ, features of lesion, and needle type |
3) Organization and structure |
|
|
Cooperation between endosonographer and cytopathologist | + | On-site cytology: possible improvement of diagnostic yield by 10–15% |
Experience, training (endosonographer, cytopathologist) | ++ | Steep learning curve |
Cytopathological methodology | + | Diagnostic improvement by immunohistochemistry and other auxiliary methods in special cases |
Quality management | + | Reduction in inaccurate and uncertain diagnoses |
4) Other factors |
|
|
Contamination: cells from the needle tract and pancreatic acinus cells | + | Differential-diagnostic problems, limitations of cytological evaluation |
Contamination: blood | + | Obstructs assessment of smears |
Influences on Diagnostic Yield
The diagnostic yield of EUS-NB is influenced by a wide variety of factors—the anatomic and structural characteristics of the target lesions, methodological aspects of the acquisition and processing of material, the level of experience and training of both the endosonographer and the cytopathologist, and the structure and organization of diagnostic centers (Table 10.12).
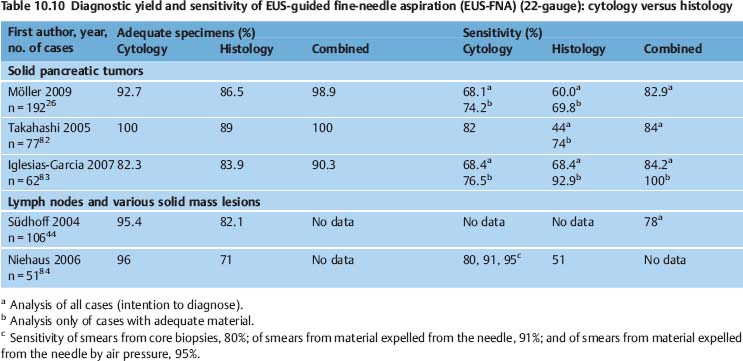
There have been very few published reports on the number of cases in which EUS-NB was not technically possible—for example, due to technical problems, interposed vessels, the anatomic location, or failed needle penetration of very hard tissue. Voss et al. reported on 99 consecutive EUS-FNAs of solid pancreatic lesions. EUS-FNA was not possible with a 22-gauge needle in nine cases. Biopsy was not carried out due to interposed vessels in four cases. Needle penetration into the tumor tissue was not possible in three cases. Anatomic problems (such as duodenal stenosis, duodenal mobility) prevented EUS-FNA in two further cases.102 Very hard tumor tissue is a negative predictive factor for the success of EUS-FNA.103 In a large series of 541 EUS-FNAs of solid pancreatic lesions, repeated endosonographic investigations turned out to be necessary. In seven of 24 patients who underwent repeated investigations, technical problems, interposed vessels, or ascites were the reason for failure of the first attempt at EUS-FNA.104 The same investigators detected periduodenal venous collaterals in 22 of 338 patients (6.5%) undergoing EUS for pancreatobiliary disorders. Twenty-one of the patients had pancreatic cancer. Venous collaterals impeded transduodenal EUS-FNA in nine patients (41%). However, the investigators succeeded in carrying out transgastric EUS-FNA of the lesion or were alternatively able to sample liver metastases in six of these patients105 (Fig. 10.2).
Fig. 10.2a–d Interposition of vessels in the needle tract.
a Peripancreatic portovenous collaterals in a patient with a small pancreatic tumor (TU, arrows).
b Small collaterals crossing the needle track in a patient with pancreatic cancer (the arrows mark the borders of the tumor). EP, endoprosthesis.
c A large mediastinal tumor adjacent to the esophagus before EUS-FNA.
d Color-coded duplex scanning shows vessels in the needle track.
Mass lesions in the head and neck of the pancreas are difficult to sample, as the echoendoscope is in a “long” position. It may be difficult in this position to extend the needle from the accessory channel, and power transmission to the needle tip is impaired due to increased friction within the angulated needle shaft. A transduodenal approach to lesions in the pancreatic head using the large-caliber Tru-Cut and aspiration needles (19-gauge) is therefore not possible in most cases.93,99,106 As arule, it is always easier to position and move the needle if the echoendoscope is straightened. If the tumor is very hard or difficult to approach, it may be advantageous to use a 25-gauge needle with a pointed tip on the stylet. In technically challenging cases, we would suggest a controlled, step-by-step procedure: firstly, the gastrointestinal wall is penetrated. The thin wall of the duodenum and esophagus is not drawn aside by the needle, and penetration is therefore easy. Particularly in the upper third of the stomach, the more robust stomach wall tends to give way to the needle. We therefore prefer to thrust the needle forward through the gastric wall with a short, rapid, and powerful movement like a dart shot. After the gastrointestinal wall has been penetrated, the position of the echoendoscope and the angle of the needle are optimized to ensure contact between the transducer and the wall and to restore ultrasound control of the needle tip. The needle tip is now positioned in close contact with the surface of the target lesion. Very short, but rapid and powerful forward movements of the needle, repeatedly if necessary, will allow the penetration of the needle even into very hard tumor tissue.
Small lymph nodes that are embedded in loose connective tissue, as well as hard subepithelial tumors, may tend to evade the needle. In this situation as well, it is advisable to penetrate the gastrointestinal wall or its superficial layers in a first step. After the needle tip has been repositioned centrally on the capsule of the node or the surface of the tumor, it is possible to identify the optimal angle for advancing the needle into the lesion. Some groups have reported encouraging results in EUS-guided biopsy of very hard pancreatic and sub epithelial tumors and of lymph nodes using an automated spring-loaded “power-shot” needle (Olympus NA-11J-KB).107
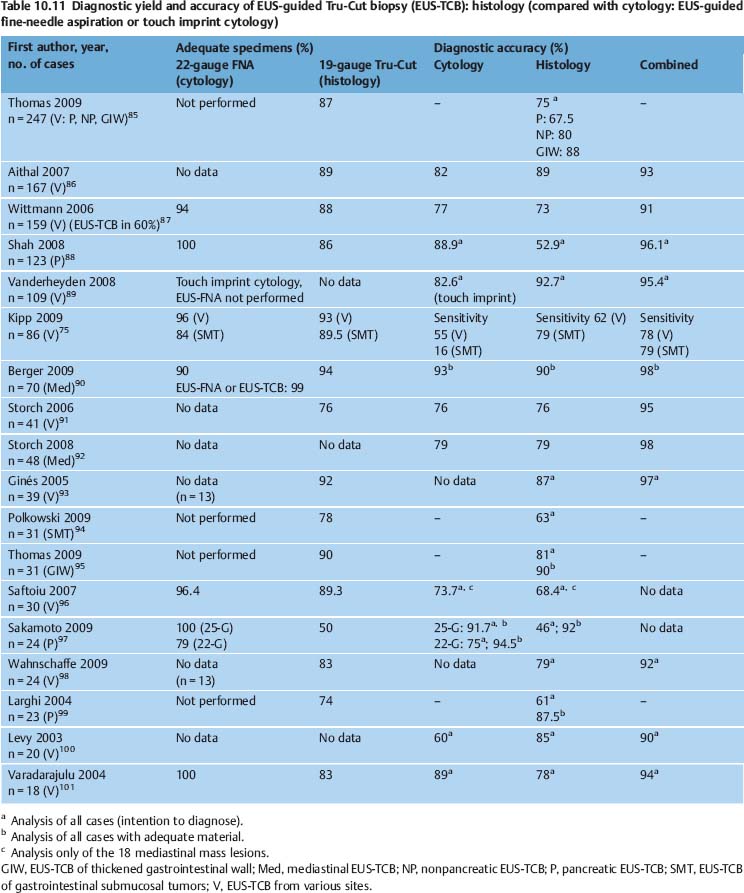
Fig. 10.3a–c Contamination of EUS-FNA specimens in some circumstances may lead to diagnostic confusion or may compromise the assessment of slides.
a Benign esophageal squamous cells.
b Benign gastric epithelia.
c Blood.
Contamination of cytological smears by epithelial cells or other cells from the needle tract is a common phenomenon in EUS-FNA. In one study, significant contamination of smears was noted in 23 of 72 cases (32%) and was reported to impede diagnostic interpretation.108 In another study, normal duodenal or gastric epithelial cells and pancreatic acini were found in 52.4% of positive and suspicious cases and 93.8% of negative cases of EUS-FNA of pancreatic lesions, respectively.21 Cellular contamination results in the aspirate being highly cellular, so that scarce malignant cells are overlooked (resulting in false-negative findings) or a neoplastic lesion is simulated (false-positive findings). Problems with differential diagnosis may arise especially in cases of chronic pancreatitis, well-differentiated carcinoma (pancreas, lymph-node metastasis), cystic lesions of the pancreas, foregut duplication cysts, and mesenchymal tumors (Fig. 10.3a, b).21,109–113 Pronounced bloody contamination as a result of inadvertent puncture of vessels near the target lesion, or when highly perfused lesions are being biopsied, also makes it difficult to assess aspirates (Fig. 10.3c).
Administration of acetylsalicylic acid (ASA), nonsteroidal anti-inflammatory drugs (NSAIDs), and low-molecular-weight heparin at prophylactic dosages has not been found to significantly increase the rate of blood contamination of EUS-FNA specimens from solid lesions. Bloody aspirates were seen in 5% of patients (one of 20) taking ASA, NSAIDs, or heparin and in 12.6% of control individuals (19 of 151).114 Applying suction during EUS-FNA of lymph nodes led to poorer results in node aspiration by increasing the risk of a nondiagnostic bloody aspirate by factor of five.115
Fig. 10.4a, b Diminutive focal metastatic infiltrations on the edge of lymph nodes (markers), which are very difficult to sample with EUS-NB.
Location of the Target Lesion
A large multicenter study by Wiersema et al.,31 as well as three other studies,20,24,116 showed that the rate of adequate specimens and the sensitivity were lower for lesions of the gastrointestinal tract wall in comparison with all other lesions. In solid pancreatic tumors, a location in the pancreatic head and accessibility of the lesion only from the descending duodenum are negative predictive factors for the diagnostic efficiency of EUS-FNA and EUS-TCB.97,103 The actual location in the upper gastrointestinal tract may influence the yield of EUS-FNA for hypoechoic subepithelial tumors. In two studies, the diagnostic yield was found to be significantly higher for gastrointestinal stromal tumors (GISTs) located in the stomach in comparison with duodenal or esophageal GISTs.67,117
Size of the Target Lesion
In pancreatic tumors and lymph nodes, the diagnostic yield and accuracy of EUS-FNA were comparable when lesions had a diameter of up to 25 mm or larger than 25 mm.42 Due to tumor necrosis in very large pancreatic masses, the negative predictive value of EUS-FNA may be unsatisfactory.15 In another study, however, the sensitivity of EUS-FNA was significantly lower in lesions less than 10 mm in size in comparison with lesions with a diameter of 10 mm or more.118 With regard to subepithelial tumors in the gastrointestinal tract, the diagnostic yield of EUS-FNA was lower for tumors < 20 mm in comparison with larger lesions.64,117 In one study, EUS-FNA of very large subepithelial tumors (>10 cm) proved to be unsuccessful in all cases.67
Focal Infiltration
Focal malignant infiltration of lymph nodes represents an obvious limitation of EUS-NB. Eloubeidi et al. used endoscopic ultrasound to examine 102 patients with esophageal cancer, searching for celiac lymph-node metastases. In patients with detectable celiac lymph nodes, the accuracy of EUS-FNA was 98%. In 14 patients in whom celiac lymph nodes were not endosonographically detectable, postoperative pathological investigation revealed the presence of 89 celiac lymph nodes with a median diameter of 5 mm (1–15 mm), 44% of which were malignant. The median diameter of metastatic infiltration in the EUS-negative celiac lymph-node metastases was only 4 mm.119 Micrometastases in lymph nodes may be missed on EUS-NB (Fig. 10.4).
Histological Type
EUS-FNA of tumors with a high density of tumor cells (such as neuroendocrine tumors and malignant lymphomas) normally produces diagnostic samples with high cellularity. By contrast, EUS-NB of ductal adenocarcinoma of the pancreas yields much less cellular material, due to marked desmoplasia and surrounding inflammation.21,112 In solid pancreatic and biliary mass lesions, the diagnostic yield and sensitivity of EUS-FNA are significantly impaired by the presence of chronic pancreatitis and marked acute inflammation.19,25,30,52,120,121 In patients who ultimately have a benign condition diagnosed (chronic pancreatitis), the rate of nondiagnostic EUS-FNA of focal pancreatic mass lesions is significantly higher than in patients with a final diagnosis of ductal adenocarcinoma.43,73 Significantly more needle passes are needed in order to diagnose well-differentiated pancreatic adenocarcinoma in comparison with pancreatic adenocarcinoma with moderate or poor differentiation.122 Obtaining diagnostic material from cystic pancreatic tumors poses a challenge for the endosonographer. Aspirating the cyst contents yields extremely hypocellular samples, but biopsying small solid parts of cystic lesions or of the cyst wall involves technical difficulties. With the exception of one study,34 therefore, the sensitivity of cytopathology alone for differentiating between mucinous and serous cystic lesions of the pancreas is reported to be unsatisfactory (27–60%) (Table 10.6; see also Chapter 18). The high cohesiveness of tumor cells limits the effectiveness of EUS-NB for assessing subepithelial spindle cell neoplasms in the gastrointestinal wall.31,123
 Technical and Procedural Variables
Technical and Procedural Variables
Number of Needle Passes
The number of needle passes needed to obtain diagnostic material varies depending on the site, size, and type of lesion, and can potentially be optimized by immediate cytological assessment of the adequacy of specimens.6,7,124,125 A systematic study of 109 patients in whom a 95% sensitivity rate for diagnosing malignancy was achieved showed that for lymph nodes and liver lesions, the number of needle passes needed for diagnosis (1.43 ± 0.66) was significantly lower than for solid mass lesions in the head of the pancreas (3.59 ± 2.29) or body/tail of the pancreas (3.10 ± 1.93). Cytological diagnosis of well-differentiated ductal adenocarcinoma of the pancreas required significantly more needle passes than diagnosis of moderate or poorly differentiated adenocarcinoma. A limitation to five needle passes in solid pancreatic lesions would have reduced the proportion of definitive cytological diagnoses by 13%.126 The results of this and other studies concerning on-site cytological evaluation show that correct diagnosis of solid pancreatic lesions may require five to seven passes. In the case of lymph nodes, tumors of the liver, and lesions of the adrenal gland, two to five passes are estimated to be sufficient42,115,116,126,127 Factors increasing the number of needle passes necessary for definitive cytopathological diagnosis of solid pancreatic tumors are: a large diameter,42 tumors with cystic parts (necrosis),122 and a setting of chronic pancreatitis.19 Interestingly, in one recent study conducted by three centers in Germany, gross macroscopic assessment by the EUS examiner of the adequacy of specimens for cytological and histological examination appeared to be sufficient to guide the number of needle passes, resulting in limitation of the number of needle passes to only one or two in 92% of patients with solid pancreatic masses. Macroscopic assessment failed in only 7% of cases for cytology and 13.5% for histology.26 Another very recent study similarly showed that in the absence of on-site cytology, only two or three passes were needed to achieve a good level of accuracy for EUS-FNA of solid pancreatic lesions.16 Various other groups with no access to an on-site cytology service have reported similar experience.30,83 The number of back-and-forth motions of the needle within a lesion needed to obtain diagnostic material has not been specifically investigated. Making ≈ 10 movements inside the lesion (with rapid inward thrusts and slow withdrawal) appears to be appropriate.6
Suction
Applying suction to the needle may increase the cellular yield of the aspirate, but on the other hand it may also increase artifacts and contamination with blood.115 One study reported no difference between suction and no suction with regard to quality and diagnostic accuracy.128 In another recent controlled study, EUS-guided fine-needle sampling with suction of solid masses increased the sensitivity and negative predictive value without increasing the overall bloodiness of each sample in comparison with the nonsuction group.129 An in vitro study of EUS-FNA of lymph nodes favored continuous suction using a 5–10 mL volume instead of intermittent suction using larger syringes (20 and 30mL).130 In practical terms, we would suggest performing one or two needle passes with continuous suction first, and adding further passes without suction if very bloody aspirates are obtained.
There are a few data suggesting that EUS-FNA with high suction (30–35 mL) may make it easier to aspirate tissue cores suitable for histological investigation. Using continuous negative pressure with a 30-mL syringe in EUS-FNA (22-gauge) of solid pancreatic tumors, Voss et al. were able to obtain small core particles in 81% of cases.102 Larghi et al. in EUS-FNA (22-G) of various solid tumors applied continuous high negative pressure suction at 35 mL with a 60-mL balloon inflation syringe and were able to obtain tissue specimens for histological examination with only one needle pass in 26 of 27 cases (96%). In two cases, in which cytological smears obtained by five needle passes using 5 mL suction failed to allow a definitive diagnosis, histological and immunohistochemical examination of the tissue cores established a diagnosis of neoplasia (recurrent thymoma, schwannoma of the gastric wall) or a benign condition (chronic pancreatitis, normal adrenal tissue).131 However, a recent randomized study comparing EUS-FNA with high suction and EUS-TCB in various solid mass lesions was not able to reproduce these promising findings. Histological core particles were obtained in only 10 of 36 cases with high-suction FNA (27.8%), in comparison with 42 of 44 cases with EUS-TCB (95.3%).132
Selection of Lymph Nodes for EUS-NB
The assessment of lymph-node metastases is a crucial factor in oncological staging. Several guidelines recommend neoadjuvant treatment in cases of nodal involvement—for instance, in esophageal and rectal cancer, and only recently in gastric cancer. For non-small cell lung cancer (NSCLC) patients, metastasis to the contralateral mediastinal hilum (N3) is a negative prognostic marker, and surgery cannot be recommended in these patients. With ipsilateral mediastinal lymph-node metastases (N2), neoadjuvant treatment may be an option. Correct nodal staging and appropriate selection of lymph nodes for EUS-NB are therefore decisively important in most patients with suspected or proven malignancies.
Fig. 10.5 Morphological scoring system for lymph nodes (adapted from D.O. Faigel).140 Three lymph-node features are assessed using a visual analoguescale, resulting in a morphology score between 3 and 15. In 93% of lymph nodes with a morphological score of 3–6, the final diagnosis was benign. Approximately 50% of lymph nodes with an intermediate morphology score of 7–12, and 79% of lymph nodes with the highest morphology score (15) proved to be malignant. For esophageal cancer, lymph nodes with a diameter of > 10 mm that were within 10 mm of the tumor, and which had a morphologyscore of 14 or 15, had a positive predictive value for malignancy of 87% when previous chemoradiotherapy was excluded.
Lymph-Node Size
Several pathological studies have shown that lymph-node size alone is not a reliable indicator for metastatic lymph-node infiltration in esophageal,133 gastric,134 colorectal,135,136 or lung cancer.137 In all of these types of cancer, metastatic lymph nodes are significantly larger than benign nodes. However, in patients with esophageal, gastric and colorectal cancer, more than 50% of metastatic lymph nodes are ≤ 5 mm in size.133,135,137 In patients with non-small cell lung cancer without metastatic lymph-node involvement, 73% had at least one lymph node that was ≥ 10 mm in diameter. By contrast, 12% of patients with metastatic lymph-node involvement had no lymph nodes larger than 10 mm.137 Despite the significant differences in diameter between metastatic and nonmetastatic lymph nodes, therefore, in gastrointestinal cancer and non-small cell lung cancer there is no definite correlation between metastatic involvement and the size of lymph nodes.
Sonomorphological Features
In addition to size, several other endosonographic criteria for malignant involvement of lymph nodes have been investigated. The standard EUS criteria typically used to assess malignant nodal involvement are based on a study of patients with esophageal cancer. In comparison with benign nodes, malignant lymph nodes are significantly more often hypoechoic, round or nearly round, and homogeneous, with a diameter ≥ 10 mm and smooth borders.138,139 In addition, a short distance from the location of the primary tumor, an advanced primary tumor, the occurrence of groups of more than five lymph nodes, and the detection of lymph nodes at specific anatomical sites such as the celiac region, should raise a suspicion of malignant lymph-node infiltration.119,140 Irregular nonshadowing, noncentral echogenic areas have been reported to correlate with coagulation necrosis and proved to be a very specific, although rather nonsensitive criterion for malignant infiltration of a mediastinal lymph node.141 By contrast, benign lymph nodes are typically hyperechoic, heterogeneous, and small. They tend to have irregular borders, a triangular or flat shape, and a regular intranodal vasculature.138–140,142
However, the diagnostic selectivity of these ultrasound criteria is rather low. One study found all of the classic criteria of malignancy in only 25% of malignant nodes.138 In rectal cancer, only 68% of malignant nodes were found to have three or more of four endosonographic features of malignancy. On the other hand, 48% of benign lymph nodes showed three echo features suggestive of malignancy.143 For lung cancer, esophageal cancer, and pancreatobiliary cancer, 4–20% of lymph nodes that did not show any of the endosonographic features of malignancy proved to have malignant infiltration.139,140,144,145 In patients with hilar cholangiocarcinoma, there were no significant differences in size or morphological appearance between benign and malignant lymph nodes.146
Assessing the size of the largest lymph node, the distance of the different lymph nodes from the primary tumor, and three morphological criteria (echogenicity, roundness, and homogeneity) on a five-point visual analogue scale, Faigel140 was able to classify lymph nodes in only 25% of 238 cases with 80–90% certainty (Figs. 10.5 and 10.6). Using this morphological scoring system, the presence of at least one lymph node ≥ 10 mm with a distance of ≥ 10 mm from the primary tumor and with a morphology score of ≥ 14 (the prevalence of this pattern was 16% of cases) had a positive predictive value of 76%. On the other hand, a morphology score of < 6 (occurring in 12% of cases) had a negative predictive value of 92%. However, 54% of the lymph nodes had an indeterminate morphological score of between 7 and 12.140
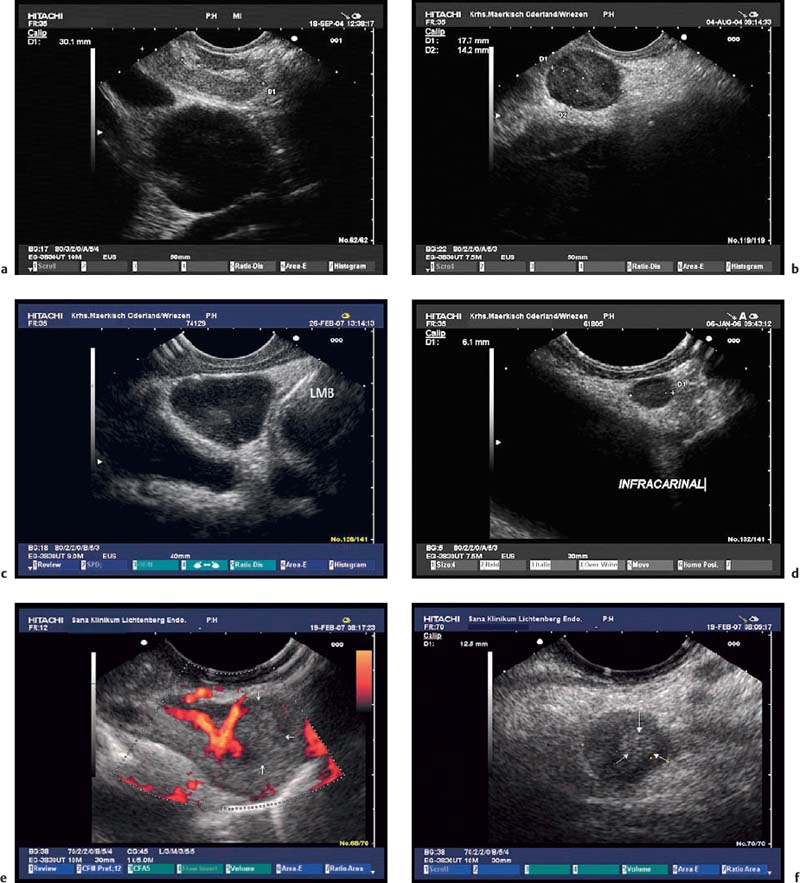
Fig. 10.6a–f Examples of lymph nodes with variable probabilities of malignant infiltration, based on the Faigel morphological score.140
a A typical benign, but large, subcarinal lymph node with distinct hilar structures (score 5).
b A typical malignant mediastinal lymph node in a patient with prostate cancer (score 13).
c A large subcarinal lymph node (score 14) in a patient without a known malignancy. EUS-FNA led to a diagnosis of sarcoidosis. LMB, left main bronchus.
d A small (6-mm) lymph node with typical malignant echo features (score 13) in a patient with non-small cell lung cancer. On the basis of the criteria used in computed tomography (CT), this lymph node would not be suspicious for malignancy. However, EUS-FNA confirmed malignant infiltration.
e, f Subcarinal and infradiaphragmatic lymph-node metastases in a patient with squamous cell cancer of the esophagus. The hyperechoic areas (arrows) are consistent with coagulation necrosis.
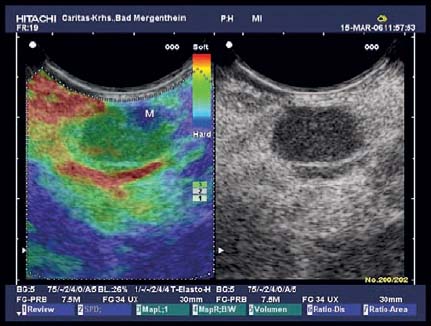
Fig. 10.7 A medium-sized hypoechoic mediastinal lymph node. Real-time elastography delineates a small area of hard tissue corresponding to metastatic infiltration (M). In this case, elastography may be helpful for targeting the needle.
In view of the high background prevalence of inflammatory and reactive lymph nodes in the mediastinum, the reliability of endosonographic lymph-node features in this location appears to be much lower than for celiac nodes, for example.140,147 In a northern European population with no malignant diagnoses, mediastinal lymph nodes were found in 62% of cases,148 while in a North American population the rate was as high as 86% of cases.149 On the other hand, abdominal lymph nodes were found in only 12% of northern European patients without malignancies—preferentially in the hepatoduodenal ligament in patients with current or previous acute pancreatitis. No celiac lymph nodes at all were detected in these patients.148 In one study, the likelihood of a mediastinal lymph node being malignant was estimated to be 2.77 times lower in comparison with celiac, peripancreatic, perigastric, and perirectal lymph nodes.147
However, the classic138,139 or modified140,145 endosonographic lymph-node features may be helpful in selecting the most appropriate node for EUS-NB. Due to the limited accuracy of these predictive factors, EUS-NB even of small nodes with only an intermediate probability of malignancy should be performed in all cases in which proof of malignant involvement of a particular lymph node station would alter the management of the patient.144 Hopefully, new ancillary endosonographic techniques (contrast-enhanced EUS, real-time elastography, EUS spectrum analysis) will not only improve differentiation between malignant and benign lymph nodes, but will also help identify areas of malignant infiltration within a lymph node (Fig. 10.7).150–156
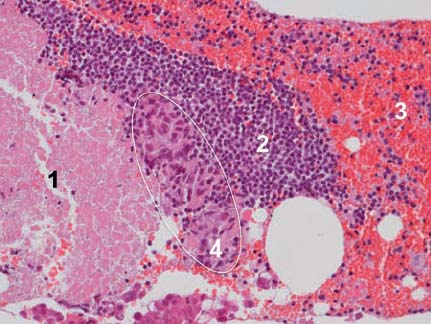
Fig. 10.8 Necrosis (1), normal lymph node tissue (2), red blood cell contamination (3), and malignant tumor cells (4, highlighted) in a specimen of mediastinal lymph-node tissue in a patient with non-small cell lung cancer (adenocarcinoma). (Hematoxylin-eosin, original magnification × 200.)
Selecting the specific needle target within a tumor or lymph node may influence the diagnostic yield of EUS-FNA. In a metastatic lymph node, for example, preexisting lymphoid tissue may coexist with viable tumor cells and necrosis (Fig. 10.8).
For large, solid pancreatic tumors, the negative predictive value of EUS-FNA was found to be considerably lower in one large study in comparison with tumors with a diameter of less than 30 mm, presumably due to tumor necrosis.15 In another study, the presence of intratumoral anechoic foci potentially representing necrotic areas was shown to be a significant predictive factor for the number of needle passes required to diagnose pancreatic cancer.122 Particularly in larger tumors, therefore, the biopsy yield is likely to be higher if the needle is passed into the periphery of the lesion, rather than into its necrotic center or into areas with a “cystic” appearance.
In lymph nodes, metastatic infiltration selectively starts in the subcapsular sinus and only later occupies the entire parenchyma (Fig. 10.4). In one randomized controlled trial, however, selective sampling of the edge of the lymph nodes did not significantly improve the likelihood of a correct diagnosis in comparison with sampling the center of the lymph nodes.115
As a practical consequence of these data, the needle should be “fanned” throughout the lesion with multiple (rapid) forward and (slow) backward motions, gradually deflecting the scope tip or alternatively using the elevator. In addition, ancillary endosonographic techniques such as contrast enhancement or elastography can be used to guide the needle in order to avoid puncture of necrotic areas.
 Cytology or Histology? Needle Type and Needle Size
Cytology or Histology? Needle Type and Needle Size
The 22-gauge needle is the standard needle for EUS-FNA. Thinner (25-gauge) as well as thicker (19-gauge) aspiration needles are less frequently used. The overwhelming majority of available studies have been conducted using 22-gauge needles and report on cytological examination of smears. Only a few centers have attempted to acquire histological material using aspiration needles (Table 10.10).26,30,40,44,51,65,82–84,102,131,157 Histological diagnosis is reasonable in all cases in which diagnosis of a specific type of neoplasia is necessary, as well as in various benign diseases. Some neoplasms, such as malignant lymphomas, gastrointestinal mesenchymal tumors, rare pancreatic neoplasms, and well-differentiated adenocarcinomas, as well as some benign diseases (e.g., autoimmune pancreatitis) are difficult to diagnose solely on the basis of cytological examination. In the case of lymph-node metastases from an unknown primary lesion, it may be crucial to specify the malignant diagnosis using immunohistochemistry or gene expression profiling.
Aspiration Needles of Different Sizes
Various recent studies concur in suggesting that in terms of diagnostic yield in the cytological diagnosis of solid pancreatic lesions and various other types of lesion, the 25-gauge FNA needle is at least equivalent14,158–160 or even superior97,161 to the standard 22-gauge needle. A 25-gauge needle may be useful particularly for highly cellular and highly vascularized targets such as lymph nodes or neuroendocrine tumors, as well as in technically difficult cases (with excessive scope bending or angulation, or very hard lesions). With 22-gauge or 25-gauge aspiration needles, satisfactory cytological smears are obtained from solid pancreatic lesions in 87–100% of cases, from lymph nodes in 65–98%, and from various other lesions in 76–100% of cases (Tables 10.6, 10.7, 10.8, 10.9). However, most of these studies have focused on distinguishing between cancer and benign disease, rather than on the ability to diagnose a specific tumor entity. To achieve a specific diagnosis in benign as well as in malignant diseases, supplementary studies such as immunostaining, molecular studies, and flow cytometry may be necessary. In this setting, taking core biopsies using 22-gauge or 19-gauge needles may be advantageous. In one prospective study of technically successful EUS-NBs using the 25-gauge aspiration needle, histological diagnosis was feasible significantly less frequently in comparison with the 22-gauge aspiration needle or the 19-gauge Tru-Cut needle.97 Using 18-gauge or 19-gauge aspiration needles, it was possible to obtain core particles for histological analysis in 69–100% of cases.40,106,157 Several groups have demonstrated that obtaining histological material is also feasible with the standard 22-gauge aspiration needle, with a comparable rate of 71–92%(Fig. 10.9, Table 10.11). In 131 consecutive EUS-FNA (22-gauge) procedures, mainly from lymph nodes, mediastinal tumors, the adrenal gland, and pancreatic lesions, our own group was able to obtain adequate material for histological assessment in 126 cases (96.2%). In four cases (3.1%), diagnosis was only possible on the basis of a histological examination of the core particles. In 18 cases (13.8%, with 11 benign diagnoses) only cytological diagnosis was possible. Immunohistochemical staining of paraffin-embedded material was necessary for specific tumor diagnosis in 10 cases (7.7%) (Wagner S, Jenssen C, Siebert C, 2007, unpublished data). Data from three centers in Germany have shown that in 166 of 192 cases (86.5%), it was possible to harvest adequate core specimens for histological assessment from solid pancreatic masses with a mean of 1.88 needle passes (22 G).26
One prospective, randomized, and controlled study compared the accuracy of EUS-FNA using the standard 22-gauge needle with that using the 19-gauge aspiration needle in patients with solid pancreatic and peripancreatic masses. The authors showed that the larger aspiration needle was advantageous for lesions in the pancreatic body and tail and for technically successful biopsies.162
19-Gauge Tru-Cut Needle
The yield of EUS-TCB has varied widely in several studies, in the range of 53–93%(Fig. 10.10, Table 10.11). In one large tertiary referral center, adequate samples were obtained in 215 of 247 patients (87%), and the overall sensitivity for diagnosing malignancy was 71%. The yield of EUS-TCB was independently predicted by the site of biopsy (transgastric procedures were better than transduodenal ones) and by the number of needle passes (more than two was better than two or fewer).85 The use of EUS-TCB is limited by technical problems, especially in targeting pancreatic head lesions85,87,97,100,106,163 and small lesions,93 and by the higher costs of the technique in comparison with aspiration needles.
In a group of patients with various lesions and in patients with mediastinal lesions, Saftoiu et al. and Berger et al. showed that specific diagnosis of malignant tumors and granulomatous disease was achieved significantly more often using the 19-gauge Tru-Cut needle than with the 22-gauge aspiration needle. This was particularly important in cases of lung cancer, mediastinal lymph-node metastases, neuroendocrine tumors, malignant lymphoma, and mesenchymal tumors.90,96 Case studies and small series have also demonstrated that EUS-TCB may be particularly valuable for diagnosing autoimmune pancreatitis,164 unexplained thickening of the gastrointestinal wall,85,95 cystic pancreatic tumors,127–129,165,188 gastrointestinal stromal tumors,75,94,96,166 malignant lymphomas,90,167 nonmalignant hepatic parenchymal disease,168,169 and rare benign and malignant lesions that are difficult to diagnose.170–173
Fig. 10.9a–c The yield of EUS-FNA using aspiration needles (22-gauge, 19-gauge). In ≈ 80% of cases, it is possible to obtain small core particles suitable for histological assessment (a reproduced with permission from 124).
a A core particle (EUS-FNA, 22-gauge needle).
b The specimen contains coagulated blood, fibrin, and small fragments of tumor tissue. Poorly differentiated cancer; material obtained from a lymph node using a 22-gauge needle. (Hematoxylineosin, original magnification × 40.)
c The core particles obtained using 19-gauge aspiration needlesare slightly larger. Squamous cell cancer of the lung; material obtained from a celiac lymph node. (Hematoxylin-eosin, original magnification × 40.)
Aspiration Needles versus the 19-Gauge Tru-Cut Needle
In 24 patients with solid pancreatic masses, the overall accuracy rates for the 25-gauge aspiration needle, 22-gauge aspiration needle, and 19-gauge Tru-Cut needle were reported to be 91.7%, 75.0%, and 45.8%, respectively.97 All other studies have shown that the diagnostic efficacy of EUS-TCB is comparable with that of EUS-FNA (Table 10.11).86,87,90–92,96,100,101,106 Interesting laboratory simulations have shown that there is very high resistance to the advancement of 19-gauge needles (aspiration needles, Tru-Cut needles) through the instrumentation channels of echoendoscopes in an upward-angulated position. The maneuverability of the endoscope and ability to angulate the Albarran lever were reduced when 19-gauge needles were used. Only the 22-gauge or 25-gauge FNA needles appeared to be suitable for insertion into the target regions if tight angulation of the endoscope or of the Albarran lever were necessary.163
A combination of cytological analysis with histological and immunohistochemical assessment is capable of enhancing diagnostic accuracy (Tables 10.9 and 10.10). This applies to “dual sampling” (EUS-FNA and EUS-TCB in one session),87,88,90,91,96 “sequential sampling” (firstly EUS-FNA or EUS-TCB and then the complementary method in a second examination, as required),86,93,98,167 performing touch imprint cytology from tissue cores obtained with EUS-TCB,89 and to the sampling of material by EUS-FNA for cytological smears as well as formalin-fixed core particles or cell blocks for histological analysis.26,51,82,83,157,174–176 Particularly in cases in which there is a suspicion of metastasis from an unknown primary, pancreatic neoplasia other than ductal adenocarcinoma, malignant lymphoma, sarcoidosis, and gastrointestinal mesenchymal tumors, we would suggest complementing cytological smears with core biopsies or cell blocks, allowing for histological investigation, immunohistochemical staining, and other ancillary examinations. Depending on the size and site of the target lesion, as well as the individual risk assessment, this is possible either with EUS-guided aspiration using a 22-gauge or 19-gauge aspiration needle or by combining EUS-FNA (25-gauge, 22-gauge) and EUS-TCB (19-gauge) in selected cases.7,124
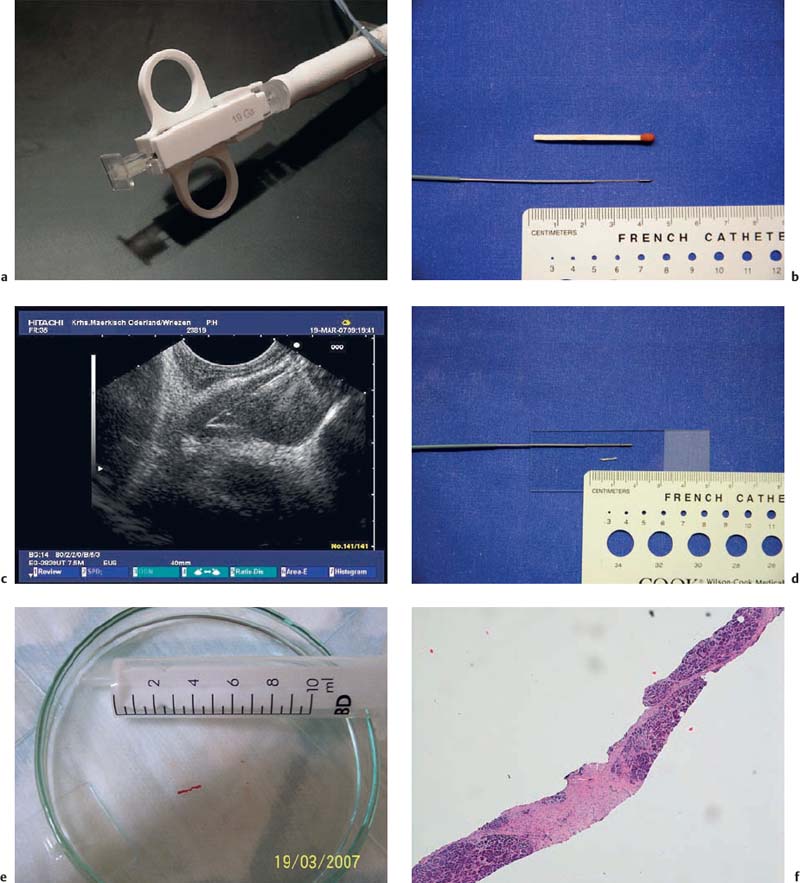
Fig. 10.10a–f 19-gauge Tru-Cut needle (Quickcore, Wilson-Cook).
a The handle of the device.
b The extended tip of the needle, with the tissue tray.
c The extended needle tip inside a subcarinal lymph node.
d The extended needle tip with a core particle from a solid lesion in the pancreatic head.
e A core particle obtained with a Tru-Cut biopsy from the lymph node.
f Microscopic assessment of the same particle, showing chronic fibrosing pancreatitis. (Hematoxylin-eosin, original magnification × 40.)
Rapid on-site evaluation (ROSE) of the adequacy of specimens and preliminary cytological diagnosis is carried out in many centers in the United States, but this is not commonly done in Europe. The aim with ROSE is to provide immediate feedback regarding the quality and adequacy of the aspirates obtained with EUS-FNA in order to optimize the efficacy of the procedure. Selected smears are air-dried, immediately stained using a quick stain, and assessed for the adequacy of tissue sampling by a cytopathologist or a cytotechnician who is present in the endoscopy suite or in an adjacent room. Rapid on-site evaluation takes ≈ 5 minutes for each needle pass. EUS-FNA is continued until diagnostic material is obtained, avoiding an unnecessarily high number of needle passes. It has been claimed that on-site cytology thus potentially shortens the procedure, improves the yield of EUS-FNA, reduces the rate of inadequate aspirates, and lowers the costs of the procedure, as well as potentially decreasing potential complications and obviating the need for repeat diagnostic procedures.6,126,177 In addition, real-time cytopathology reviewing allows an immediate preliminary diagnosis. The preliminary diagnoses resulting from ROSE are highly reliable; in three large studies, they differed from the definitive diagnoses in only 5.8%,178 8.4%,179 and 11.5%180 of cases, respectively. Depending on the preliminary diagnosis, it is also possible to allocate material for ancillary studies (microbiological culture, flow cytometry, cell-block techniques, immunochemistry, and molecular studies) if necessary.181
Although several experts advocate the use of on-site cytopathology, there are few data to support the claim that EUS centers should allocate resources for ROSE. Klapman et al. retrospectively compared the diagnostic yield of EUS-FNA samples between two academic hospitals that did and did not have facilities for performing rapid on-site cytological interpretation. The EUS-FNA procedures at both institutions were performed by the same endoscopist. Patients in the hospital with a cytopathologist were more likely to have a definitive diagnosis of malignancy and were less likely to have inadequate sampling or require a repeat procedure.182 However, there have as yet been no prospective, randomized, and controlled trials to confirm that ROSE improves the sensitivity and/or effectiveness of EUS-FNA. Some retrospective comparative studies have reported that ROSE reduces the number of needle passes needed for diagnosis, improves the rate of conclusive diagnoses, and increases the total diagnostic yield of EUS-FNA by 10–15%.16,182,183 In a large multicenter study, however, only the negative predictive value of EUS-FNA for extraintestinal mass lesions proved to be significantly better in two centers with on-site cytology in comparison with two other centers in which ROSE was not available. The sensitivity, specificity, accuracy, and positive predictive value of EUS-FNA of lymph nodes, extraintestinal mass lesions, and mural lesions in the gastrointestinal tract did not differ between centers with or without on-site cytology.31
ROSE also has several shortcomings. EUS has expanded from being available at only a few academic centers to community hospitals and outpatient endoscopy units. In Germany, for example, there are now some 800 centers providing EUS facilities, representing approximately 40% of all hospitals in the country.1 The majority of these smaller centers are not capable of providing on-site cytology at the time of each EUS-FNA. Despite potentially reducing the number of needle passes needed, ROSE may slightly increase the costs of EUS-FNA and the procedure time required for it. In addition, an on-demand cytopathology service creates several challenges for the cytopathologist with regard to work flow, time management, resource allocation, and reimbursement of expenditure. Alternative options are therefore still being investigated—for example, telecytopathology for on-site cytology diagnoses, or assessment of the adequacy of specimens by cytotechnologists, EUS assistants, or endosonographers without the need for a cytopathologist to attend. The results of these studies have been inconsistent. For example, the ability of cytotechnologists or trained endosonographers to carry out on-site evaluation of the adequacy of smears obtained with EUS-FNA is a matter of controversy. In one study, three experienced endosonographers who had completed formal cytology training with the cytopathologist were found to have a poorer ability to interpret the adequacy of specimens in comparison with cytotechnologists.184 In a recent study, by contrast, ROSE conducted by the endosonographer himself proved to be as effective as ROSE by a cytopathologist.185 In solid pancreatic masses, gross visual assessment of smears by trained EUS assistants or by cytotechnologists was shown not to be as reliable as ROSE.186 On the other hand, another study showed that assessment of the gross appearances of FNA specimens reliably predicts the adequacy of the samples.187 For specific types of lesion (e.g., cystic pancreatic lesions), the gross visual appearance of the aspirated material may also provide valuable additional information toward a specific diagnosis.187,188 One retrospective study recently suggested that telecytopathology may be an appropriate substitute for ROSE, showing that it was statistically equivalent in accuracy to rapid on-site cytopathological examinations of EUS-FNA specimens from pancreatic lesions.189
Experience, Training, and Dialogue
The skill and experience of both the EUS examiner and the cytopathologist play a key role in the diagnostic yield of EUS-NB. A large multicenter study showed that the accuracy of EUS-FNA of lymph nodes, extraintestinal mass lesions, and mural lesions of the gastrointestinal tract improved with time and growing experience. In the period from January 1994 to February 1995 (92%, n = 226) accuracy was significantly higher than in the initial period from January 1991 to December 1993 (80%, n=193).31
On the basis of the published learning curve for a single endosonographer with limited experience (with 130 independent EUS examinations) in EUS-FNA of pancreatic lesions, it appears that at least 30 EUS-FNA procedures are required before the sensitivity for diagnosing pancreatic adenocarcinoma reaches 80%, and that 50 procedures are needed to constantly achieve a sensitivity of ≈ 90%.190 However, the learning curve also needs to continue after this initial experience. Approximately 150 EUS-FNAs of pancreatic lesions proved to be necessary to gain comprehensive expertise in EUS-FNA of the pancreas, not only with regard to a reliably high accuracy, but also in terms of safety and efficiency.191
Another study impressively emphasized the invaluable significance of hands-on training. Mentoring was provided by an endosonographer with 12 years’ experience in endoscopic ultrasound (more than 10,000 EUS examinations and more than 2500 EUS-FNAs). A very brief training period substantially improved the performance of three endosonographers with very limited experience in EUS-FNA (only 20 procedures in a 17-month period, with no supervision).192 These data provide support for the American Society for Gastrointestinal Endoscopy (ASGE) recommendation that at least 150 supervised procedures, including 50 EUS-FNAs (25 pancreatic and 25 nonpancreatic) have to be performed in order to achieve comprehensive competence in all aspects of EUS.193
The diagnostic success of EUS-FNA depends of course not only on the endosonographer’s experience, but also on the cytopathologist’s expertise. In comparison with CT-guided and TUS-guided biopsies, the material obtained with EUS-NB has several special features—for example, contamination by gastrointestinal epithelia from the needle track.113,124 This may cause difficulties for general pathologists and cytopathologists who have no experience in EUS-FNA. The problems can be overcome through training courses and dialogue. One recent study showed that following a short but intensive training session, experienced general pathologists who had little experience with EUS-FNA followed a steep learning curve, resulting in markedly improved reproducibility of their cytological diagnoses from EUS-FNA of mediastinal lymph nodes.194 In a gastroenterological tertiary referral center, the percentage of nondiagnostic specimens from EUS-FNA similarly decreased significantly from 27% to 6% after a 1-monthjoint training period in EUS-FNA cytology for cytopathologists and endosonographers.81 Experience at a large academic EUS center clearly demonstrates that the usual learning curves for EUS examiners and cytopathologists in EUS-FNA depend on the tissues that are targeted and are much steeper for lymph nodes than for pancreatic lesions. In a high-volume center in the United States, an acceptable rate of less than 10% for nondiagnostic (inadequate, atypical, or suspicious) findings and false findings (false-negative and false-positive cases) was achieved after 48 EUS-FNAs of mediastinal lymph nodes had been carried out, in comparison with 171 (nondiagnostic) and 186 (misdiagnosed) pancreatic EUS-FNAs.195
Whatever approaches to EUS-NB a specific center prefers, continuous and direct dialogue between the endosonographic examiner and the cytopathologist is the cornerstone of diagnostic success. The major prognostic and therapeutic impact of cytopathological diagnoses resulting from EUS-FNA or EUS-TCB requires a sense of shared responsibility.7,124,125,181,196
Handling, Preparation, and Processing of EUS-NB Specimens
The aspirate is initially expressed from the needle by slowly advancing the stylet. Once the stylet has been completely advanced onto the tip of the needle, a 2–10-mL air-filled syringe should be used to express further material. After the last needle pass has been completed, the final step should be to flush the needle with saline to expel all remaining material from it. There are several promising approaches to preparing the material obtained with EUS-FNA, and each of them has its own advantages and drawbacks. To obtain the maximum diagnostic information, complementary use of different preparations is worthwhile. For cytological analysis, smears (air-dried or alcohol-fixed) or liquid-based preparations can be used alternatively. For histological and immunohistochemical examinations of paraffin-embedded sections, small core particles and cell blocks can be used. Aspirates from different lesions should be carefully separated from each other. Unambiguous marking of each sample or smear is mandatory.181
Cytological Smears
Cytological smears can be prepared by the endoscopist, by a trained endoscopy nurse, by a cytotechnologist, or by a cytopathologist. The smearing technique requires appropriate training, and is described in detail in Chapter 11. Properly produced smears are decisive for establishing an unequivocal diagnosis. Appropriate fixation of cytology specimens is crucial to the preservation of the cellular components. Depending on the planned staining technique, two types of smear can be prepared: air-dried or alcohol-fixed.181 The specific fixation method used determines subsequent staining and also modifies the cells in various ways. Fixation and staining consequently highlight different cytological details (Table 10.13). Air-dried smears are typically stained using a Romanowsky stain—for example, May-Grunwald-Giemsa (MGG) or Diff-Quick (Chapter 11). Air-dried smears allow rapid staining (Diff-Quick or Hemacolor), followed by on-site assessment of smears. Immunocytochemistry in particular is possible using air-dried material. Other stains, such as the periodic acid-Schiff (PAS) reaction, Alcian blue, Ziehl-Neelsen, or Gram can be used, depending on the diagnostic needs in each specific case. If a smear is air-dried, it should not be placed in an alcohol fixative and must not be exposed to formalin or formalin fumes, which alter the cells and interfere with the staining reactions. Romanowsky stains are commonly used to examine blood smears and bone-marrow aspirates, and are therefore also suitable for evaluating lymph-node aspirates. Alcohol fixation preserves nuclear features well and is typically followed by Papanicolaou (Pap) or hematoxylin-eosin (H&E) staining. It is important to prevent air-drying before alcoholic fixation.
Liquid-Based Monolayer Preparations
Preparations such as Autocyte, ThinPrep, and Pap-Spin are interesting alternatives for processing samples obtained by EUS-FNA. The sample is placed in a specific transport and fixative medium. In an automated process, slices are prepared with a uniformly monolayer cell dispersion. The theoretical advantages of these preparations are that they do not depend on an individual smear technique; they eliminate mucus, erythrocytes, and protein precipitates from the sample; and there is highly consistent cell preservation, with an increase in diagnostic material with high cellularity on the slide and optimal assessment of single cells. In addition, ancillary studies (immunocytochemistry, DNA analysis) are possible using these monolayer preparations.181 However, interpretive difficulties may arise as a result of disaggregation of cell clusters, loss of characteristic extracellular features (e.g., mucin in mucinous pancreatic neoplasms), and alteration of some cytological features. There is as yet only limited experience with liquid-based preparations of material obtained by EUS-FNA. Two nonrandomized studies have shown that liquid-based preparations are less sensitive in comparison with conventional smears after EUS-FNA of pancreatic lesions and lymph nodes.197,198
Cell Blocks
Cell blocks can be prepared from aspirated fluids (cyst contents, ascites, pleural fluid), from the needle rinse, and from part of the material collected in the proprietary fixative used for liquid-based preparations. They can be useful adjuncts to smears for establishing a more definitive cytopathologic diagnosis.176 The effectiveness of cell blocks lies in the availability of diagnostic material for further histological examination, histochemistry, and immunohistochemistry for better typing of malignancies, and for identifying the infectious cause with microbiologic stains. A wide range of histological fixatives has been used for cell-blocks—primarily, neutrally buffered formaldehyde solution, Bouin solution, picric acid fixative, Carnoy fixative, and ethanol. Following a series of centrifugations, the resulting pellet undergoes routine formalin fixation, paraffin embedding, and sectioning for standard hematoxylin-eosin staining and a variety of other stains and immunohistochemical studies.181 Sufficient material for cell-block preparation can be obtained in more than half of EUS-FNA procedures.36,42 The overwhelming majority of studies have reported an increase in diagnostic information, particularly in cases of neuroendocrine and mesenchymal tumors, cystic lesions, and lymphoma.21,28,34,41–43,45,62,74,112,174 Additional passes for cell blocks are highly recommended for lesions that may require ancillary studies, whenever core particles are not obtained by EUS-FNA.
Histology and Immunohistochemistry
Core particles that are sufficient fortraditional histological techniques are obtained by EUS-TCB in 80–90% of cases (Table 10.11). However, depending on the specific method used to handle the samples, this can also be done with EUS-FNA using 22-gauge and 19-gauge needles (Table 10.10). After the material has been expelled from the aspiration needle onto slides, a gross visual inspection of the slides is carried out, looking for small tissue particles and cylinders of coagulated blood. These particles and cylinders are withdrawn from the slides using a small needle and placed in a formalin solution. The remaining material is smeared onto the slides and air-dried or fixed with alcohol. In our experience, these cylinders consist not only of blood and fibrin clots, but very often also contain small tissue fragments that can be processed for traditional histological assessment and ancillary studies (Fig. 10.9).
Molecular Studies and Flow Cytometry
Several studies have shown that molecular analyses are feasible with samples obtained using EUS-FNA.199 Material for molecular studies has to be protected from potential RNA degradation by immediate cooling and incubation with a specific medium containing ribonuclease inhibitors. For flow-cytometric analysis, material from approximately three additional needle passes is collected into either heparinized, phosphate-buffered saline or a tissue culture transport medium containing calf serum, such as Hanks solution or Roswell Park Memorial Institute (RPMI) medium. Flow cytometry of EUS-FNA specimens has been shown to improve the diagnosis of non-Hodgkin lymphoma significantly.44,59,200–203
Microbiology
Case studies have shown that EUS-FNA is useful for obtaining samples suitable for fungal and bacterial cultures.204,205
Reliability of Diagnoses and Diagnostic Pitfalls
There are several possible reasons for misdiagnoses in the cytopathological interpretation of material obtained with EUS-NB: sampling error, inadequate sample processing, errors in interpretation by the cytopathologists, and insufficient dialogue between the clinician, endosonographer, and cytopathologist (Table 10.14). There are few published data relevant for assessing the scale of difficulties and errors occurring in cytopathological interpretation of material obtained with EUS-FNA.
 Reproducibility of Cytopathological Diagnoses
Reproducibility of Cytopathological Diagnoses
Only two studies have analyzed the reproducibility of cytopathological diagnoses of specimens from EUS-FNA, both documenting good to excellent diagnostic agreement between experienced cytopathologists in assessing EUS-guided fine-needle aspiration samples from mediastinal lymph nodes and mass lesions. In one study, the kappa value for diagnostic agreement between two experienced cytopathologists was 0.88.76 Another study compared the level of diagnostic agreement regarding materials obtained with EUS-FNA and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) between pathologists with different levels of experience before and after an educational session. There was excellent reproducibility between the two observers, who were both highly experienced in assessing mediastinal fine-needle aspirates (K = 0.89 and 0.87). The reproducibility of diagnoses made by experienced pathologists with little experience in EUS-FNA and EBUS-TBNA improved markedly following a training session.194
Three studies showed a very high level of agreement between preliminary on-site cytopathological diagnoses and final diagnoses. The on-site diagnosis and final diagnoses differed in only 51 of 656 cases (8.4%),179 80 of 1368 cases (5.8%),178 and 52 of 485 cases (11.5%).180 Scant cellularity or nondiagnostic material on the slides interpreted on site were the most frequent reasons for discrepancies, accounting for 21 of 51 (41%)179 and 38 of 80 (47.5%)178 cases, respectively, of disagreement between preliminary and final diagnoses. Cytopathological interpretation was difficult or discrepant in only 72 of 1934 cases (3.7%) and was ultimately possible using ancillary studies or intra-departmental/extradepartmental consultations in 40 (56%) of the 72 difficult cases.178,179 The level of agreement between preliminary on-site diagnosis and final cytological interpretation was significantly better for malignant than for nonmalignant diseases (98.8% vs. 67.2%). Upgrading of preliminary diagnoses occurred more often than downgrading.180
Factors | Relevance | Problem |
1) Target organ/target tissue |
|
|
Anatomic site | + | Problems in obtaining diagnostic material: pancreatic head (especially uncinate process) and neck, gastrointestinal wall lesions; interposition of vessels |
Biological tissue characteristics | + | Problems in obtaining diagnostic material: benign lesions, well-differentiated ductal pancreatic adenocarcinoma |
Type of neoplasia | ++ | Problems in obtaining diagnostic material: subepithelial gastrointestinal tumors, pancreatic cancer, cystic lesions |
Vascularization of target lesion | + | Bloody contamination of aspirates |
Size of lesion | + | Problems in obtaining diagnostic material: very small lesions, very large and necrotic mass lesions |
2) Methodological aspects |
|
|
Type and diameter of needle | + | Influence on: diagnostic yield (cytology, histology), technical feasibility |
Aspiration | + | Influence on: cellularity, yield of histological material, bloody contamination |
Number of needle passes | ++ | Dependent on target organ and tumor type |
3) Organization and structure |
|
|
Cooperation between endosonographer and cytopathologist | + | On-site cytology: possible improvement of diagnostic yield by 10–15% |
Experience, training (endosonographer, cytopathologist) | ++ | Steep learning curve |
Cytopathological methodology | + | Diagnostic improvement by immunohistochemistry and other auxiliary methods in special cases |
Quality management | + | Reduction in inaccurate and uncertain diagnoses |
4) Other factors |
|
|
Contamination: cells from needle track and pancreatic acinus cells | + |
 Technical Problems
Technical Problems
 Contamination
Contamination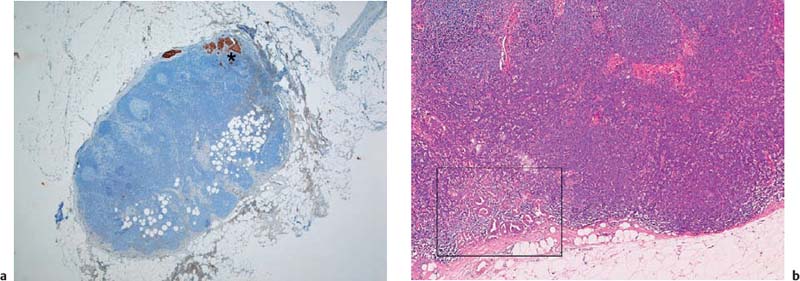
 Features of the Target Lesion
Features of the Target Lesion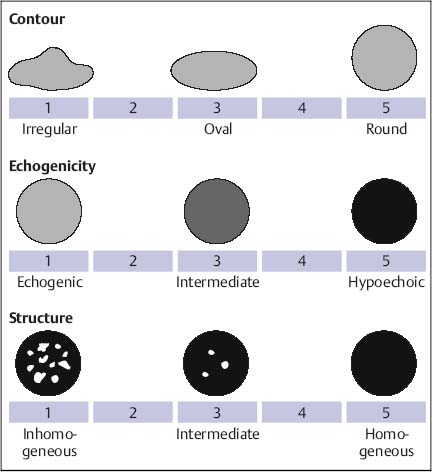
 Targeting
Targeting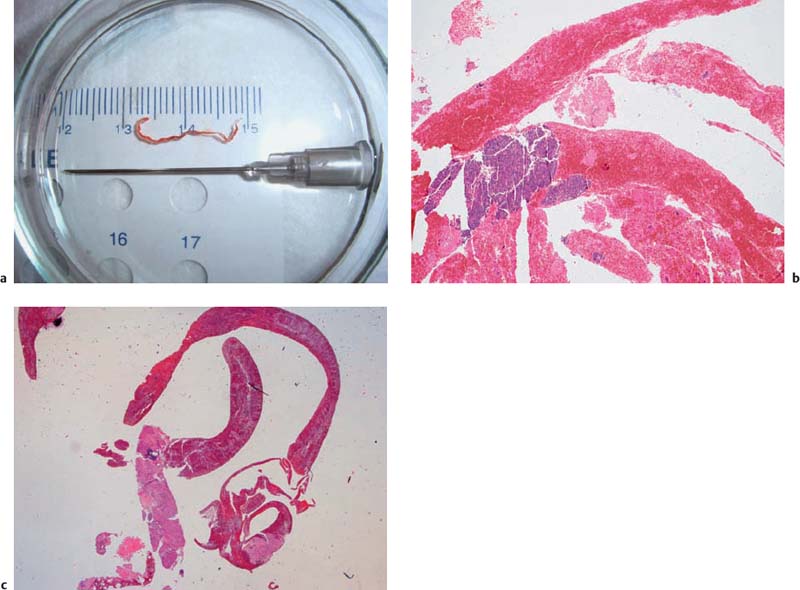
 Cytology and/or Histology
Cytology and/or Histology