Localization: ES is as frequent in flat and short bones as it is in long bones. Among the appendicular skeleton, the most common localization is the femur, followed by the tibia, humerus, fibula, and forearm bones. It is rare in hands and feet. In the trunk, the most frequent localization is the pelvis, followed by vertebrae and sacrum, scapula, ribs, and clavicle. It is rare in the skull. In long bones, tumor is common in the midshaft, but it may involve a larger portion or even the entire bone.
Clinical: Pain is the earliest symptom (intense and radiating if nerve impingement is present, as in sacral and vertebral locations). Swelling is also a common and early sign. Occasionally, however, no soft tissue mass is appreciated, even for a long time. Low-grade fever may be associated. An increase of serum HDL and erythrocyte sedimentation rate is frequently seen, sometimes associated with leukocytosis and anemia.
Imaging: The imaging presentation is variable: the most frequent is a permeative, infiltrative, poorly defined osteolysis that can also be more destructive, uniform, or vaguely trabeculated. Increased bone radiodensities may associate (bone necrosis and/or deposition of reactive bone). The cortex is almost regularly breached by the tumor or destroyed, and a radiolucent extraosseous tumor mass is usually present. Particularly in flat bones, this mass may be prominent, in contrast with a minimal cortical osteolysis. Rarely, however, the tumor remains intramedullary for a long time. Periosteal reaction is common in the diaphyses of younger patients. The most typical, but infrequent, and unspecific sign is an onionskin appearance, leading to a fusiform enlargement of the shaft. Rarely ES is predominantly periosteal, with erosion of the outer cortex. CT defines alterations of bone and of the extraosseous mass (density similar to muscle). MRI is the best way to study tumor extension in marrow spaces (better demonstrated by STIR technique). Isotope scan and PET scan are hot and may occasionally show skeletal mets at presentation. Typically, extension of ES, as shown by MRI, CT, and isotope scan is much more than what appears on X-rays.
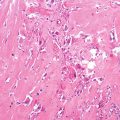
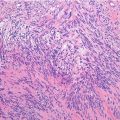
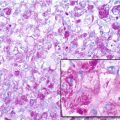
Histopathology: ES is very soft and grayish, and when incised it produces a milky fluid. It may be frankly hyperemic or hemorrhagic. Necrosis is common, particularly in the center with a semiliquid appearance, similar to that of purulence. Microscopically the tumor is uniformly made of sheets of small round cells closely packed and without matrix. Cells have a rather monotonous appearance. Cytoplasm is scarce, pale, granular, and clear to eosinophilic, with poorly defined limits. The nuclei are round/oval, with a distinct nuclear membrane and powder-like chromatin, with one or more tiny nucleoli. Mitotic figures are usually rare. Reticulin fibers are lacking between cells but may enwrap cell islands. Blood vessels are numerous and thin. PAS diastase staining (particularly after alcohol fixation) shows intracytoplasmatic glycogen, even very abundant, in the vast majority of cases. Immunohistochemistry shows positivity to vimentin and specific antigens. Electron microscopy shows glycogen in the cytoplasm. Sometimes cells form a rosette pattern (with nuclei at the periphery and cytoplasmatic projections toward the center). Pseudorosette appearance can also be related to necrosis in the center of a cellular nest. When immunohistochemistry shows positivity to S-100 and to NSE, and electron microscopy shows dendritic processes, neurotubes, and dense-core neurosecretory granules, the tumor is labeled as PNET. Necrosis is very frequent and extensive. In areas of necrosis, leukocytic infiltration may be prominent, and it should not be confused with osteomyelitis. The tumor diffusely permeates the marrow spaces, before initiating bone destruction. There is a prominent neovascular and inflammatory response, while fibro-osseous reaction is not able to provide a significant encapsulation. At the edges of the tumor, there are frequent satellites in and well beyond the reactive zone, and even skips.
Course and Staging: The tumor usually displays an aggressive growth. There are, however, cases where ES remains intraosseous causing only discontinuous pain for a time ranging from 1 to several years. ES frequently has a multiple metastatic spread to the lungs, skeleton, lymph nodes, and brain, and even a multicentric origin has been postulated. Because of this, Enneking proposed a modified special staging for this tumor: EW I, solitary intraosseous; EW II, solitary extraosseous; EW III, multicentric, skeletal; and EW IV, distant metastases. The most frequent stage at presentation is EW II (70 % of cases). Metastases at presentation are about 20 %.
Treatment: ES is sensitive to chemotherapy and radiotherapy, but surgery plays an essential role in treatment of the primary tumor. Thus, treatment is based on a combination of chemotherapy (always), surgery (whenever feasible), and radiotherapy (alternative to surgery or associated to less than wide or radical surgery). Chemotherapy is given pre- and postoperatively and is used as a combination of at least 4 drugs, the most effective being cyclophosphamide, ifosfamide, adriamycin, vincristine, dactinomycin D, and etoposide. Preoperative ctx, in responsive patients, which are the vast majority, causes an extensive necrosis of tumor cells. Because ES cells do not produce any matrix and disappear short after necrosis, effective chemotherapy determines a remarkable shrinkage of the tumor, plus recession of inflammation. Local treatment (surgery alone, surgery and radiotherapy, or radiotherapy alone) is planned according to stage and several individual factors (age, locations, radiological response to ctx) and by balancing the risk of local recurrence against functional loss secondary to local treatment.
Prognosis: Before the use of chemotherapy, long-term survival was less than 10 %, also in localized cases. Presently, with multimodal treatment, the same figure is around 60 %. Local recurrence occurs in about 20 % of cases treated with radiation (without surgery) plus chemotherapy. It is rare after wide surgery plus chemotherapy (without radiation). Metastases, usually multiple or disseminated, appear most frequently in the lungs, followed by the skeleton and the lymph nodes. Treatment with aggressive chemotherapy and bone marrow transplantation or with whole-body irradiation is still experimental. A more favorable prognosis can be expected in distal appendicular localizations, small tumor volume, normal ESR and HDL values, use of adequate surgery, and good to complete response to preoperative chemotherapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree