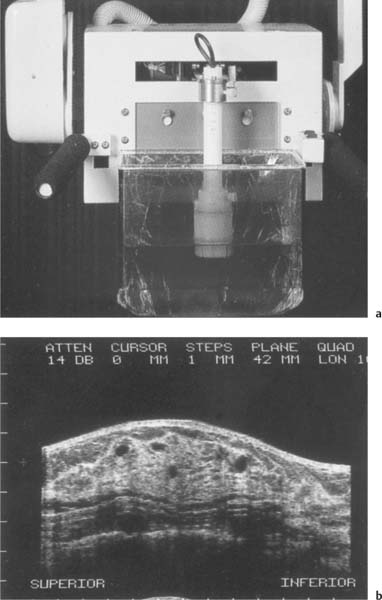
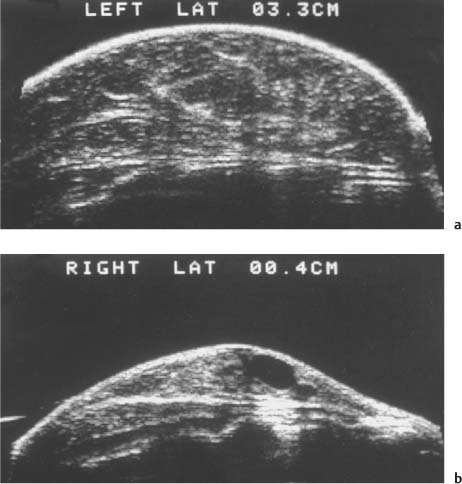
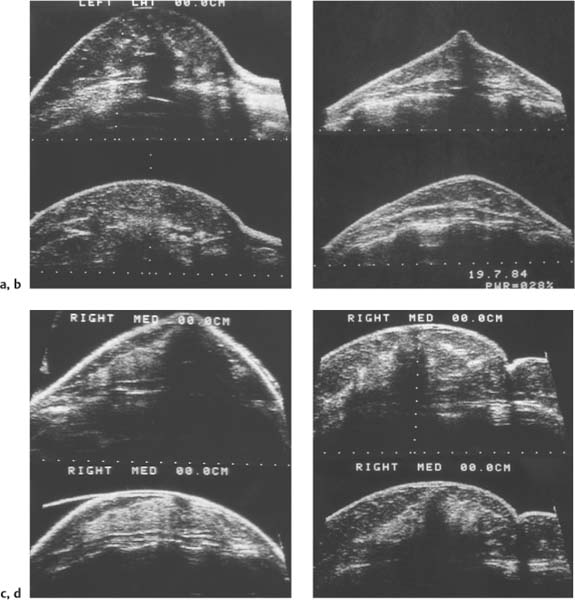
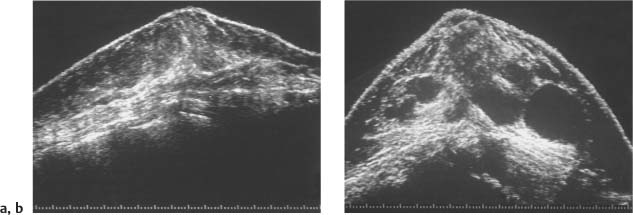
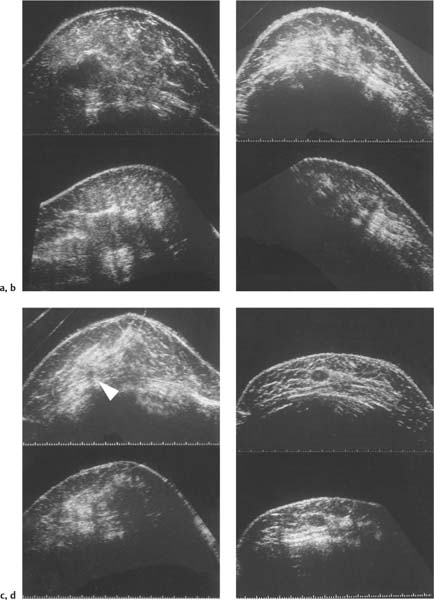
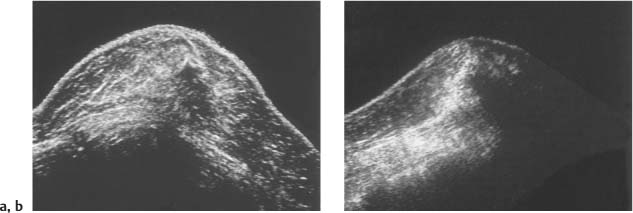
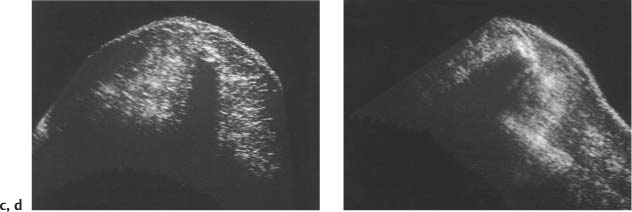
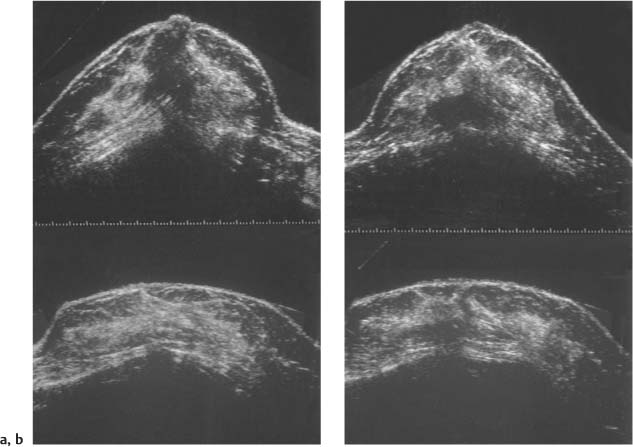
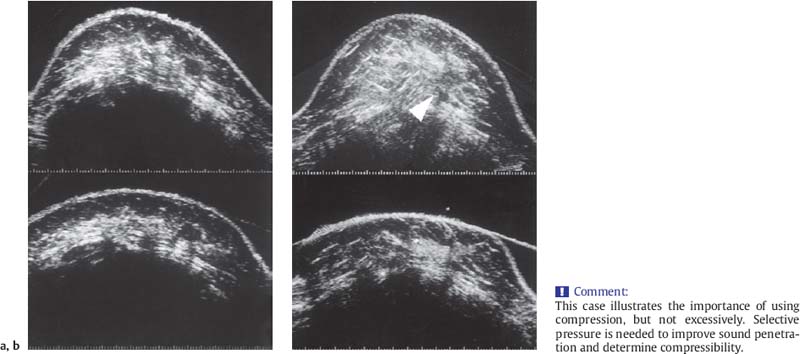
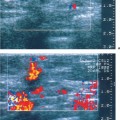
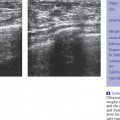
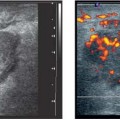
2 Examination Technique: Historical and Current A recognition of the inherent limitations of mammography in depicting masses in dense fibroglandular tissue has sparked renewed interest in adjunctive modalities that might supplement mammography for breast cancer screening. Most important currently are MRI and ultrasound, and both are subjects of intense, active research. Various devices for ultrasonic breast imaging were developed during the 1970 s and 1980 s. The single-focus real-time scanners and handheld mechanical transducers used at that time were unable to provide good lateral resolution. The design principle of water-path scanners is based on mechanical single-crystal transducers. These probes have a large diameter with a correspondingly large focusing radius. Thus, the sound beam narrows gradually over a long distance and provides good focusing over a longer range. This simple principle made it possible to achieve uniform image quality within the region of interest. An essential component was the water path interposed between the transducer face and the breast, accomplished either by using a water bag or by immersing the breast in water. In this technique the patient is examined supine. A small water tank sealed at the bottom with plastic film is placed onto the breast. A single-crystal transducer is introduced into the tank from above and is motor-driven along a linear path (Fig. 2.1 a, b), producing static longitudinal or transverse images. Image generation was relatively slow, however, and a complete survey of the breast was time consuming. The real-time examinations relied upon currently for lesion characterization and ultrasound-guided needle procedures were almost impossible. It was also difficult to obtain good contact at the periphery of the bag, particularly at the lateral aspect of the breast and the axilla. Scanning large breasts was also challenging. Thus, although this technique provided excellent anatomic detail, these systems are no longer available for clinical use in the United States. Fig. 2.1 a, b Mechanical transducer used with a water bag for breast scanning in the supine patient. a The motor-driven transducer is moved in a linear pattern and provides sectional images of the entire breast. b Example of breast cysts. Fig. 2.2 a, b Water-path immersion scanner. a System 1 (Ausonics, Australia). The breast hangs through an opening into the water tank. Four motor-driven transducers inside the tank are moved in a pattern that systematically surveys the entire breast. b View of the mechanical sector transducers inside the tank. The four transducer images are superimposed to produce a compound scan, or an image reconstructed by superimposing multiple scans from different directions. Alternatively, simple scans can be obtained by activating only one transducer. Although this and other older automated scanners are no longer available, there has been a revival of interest in automated scanners suitable for supplemental breast cancer screening of women with mammographically dense fibroglandular tissue and high risk of breast cancer. Immersion scanning was once widely practiced, and as early as the 1970 s it was used for clinical studies at many centers throughout the world. The patient lay prone on a table over a large water tank (Fig. 2.2 a, b). The breast entered the tank through an aperture similar to that found in prone tables used today for stereotactic biopsies, and hung freely in the water. Motor-driven transducers on the floor of the tank generated sector scans in sagittal or transverse planes. The transducers could be moved horizontally by remote control to scan the breast systematically, section by section, to obtain complete breast coverage. Some scanners had only one sector transducer which provided simple scans similar to those in real-time imaging but depicting a representative section of the whole breast (Fig. 2.3 a, b). These scanners were relatively easy to operate. Usually, some degree of breast compression was applied to improve sound penetration and eliminate refraction artifacts (Fig. 2.4 a–d). Compression alters sound propagation and can be used to distinguish artifactual attenuation from acoustic shadowing associated with carcinoma and other breast lesions, such as stromal fibrosis. Higher-end systems were equipped with multiple transducers that could scan the breast from different directions. The images were then superimposed to produce a composite (compound) scan. A simple scan can only demonstrate structures that are insonated at a favorable angles, but a compound scan allows many more surfaces to be presented perpendicular to the transducer. As a result, compounding provides a sharper and more detailed depiction of breast anatomy (Fig. 2.5 a, b). Fig. 2.3 a, b Breast anatomy demonstrated by immersion scanning with one transducer (simple scan technique). The breast hangs freely into the water bath, providing an expanded view of anatomic structures. Compression alters but can improve the appearance of the image. Optimum compression was used in these examples. a Fatty breast. b Breast cysts. Acoustic enhancement and shadowing are posterior features included in ultrasound characterization of masses. The multiple angles of insonation in compound scanning caused reduced conspicuity of both enhancement and shadowing in the water immersion technique just as these features are less perceptible in real-time spatial compounding. In immersion breast examinations, it was common to obtain both simple and compound scans. The compound scan provided better anatomic definition and marginal detail of individual focal lesions, while a simple scan revealed the associated posterior features. (Fig. 2.6 a–d). Additional information was obtained in simple scans by selectively activating transducers at different positions and scanning the breast from variousangles (Fig. 2.7 a, b). Fig. 2.4 a–d Comparison of noncompression and compression immersion scans. As the examples show, slight compression is usually necessary to improve image quality. a Fatty breast. This breast is ≤ 25% dense (BIRADS category 1 density for mammography). The noncompression image (top) shows a central acoustic shadow. When light compression is applied (bottom), the acoustic shadow disappears and the central portion of the breast can be evaluated. b Breast with scattered fibroglandular densities, 26–50% dense (BI-RADS category 2 density for mammography). Without compression (top), a dense subareolar acoustic shadow is seen despite the paucity of breast parenchyma. Compression eliminates this artifact and reveals the continuity of breast architecture below the nipple. c Adolescent breast is extremely dense—category 4 BI-RADS density (76–100%). The non-compression image (top) shows a broad subareolar acoustic shadow. Compression eliminates the shadow obscuring the central breast, permitting evaluation. d Breast carcinoma measuring 2.5 × 3cm. The acoustic shadow caused by desmoplasia persists when compression is applied. The persistence helps confirm a hypoechoic, shadowing mass with irregular, ill-defined margins. Compression would have eliminated refraction artifacts at glandular interfaces, and there is little change in the appearance of the mass following compression. Fig. 2.5 a, b Compound scan: need for scanning in at least two orthogonal planes. a Normal appearance of an involuted breast, sagittal scan. b Multiple cysts in the plane of the nipple, transverse scan. In immersion scanning the breast was usually not compressed, but as in simple scanning there are instances where compression enhanced sound penetration and improved the compound image. This was accomplished by stretching a sheet of film across the tank to flatten the breast. Compression was necessary to confirm a suspected tumor, as Figs. 2.8 a, b and 2.9 a, b illustrate. Water-bath immersion is outmoded because of cost, large numbers of images to review, and poorer spatial resolution compared with current real-time scanners, but the classic water-bath scan still has value as a teaching tool. Fig. 2.6 a–d Comparison of compound and simple scans. a Invasive breast carcinoma, 3 × 4 cm. Applicable to small field of view, high-resolution hand-held ultrasound as well as to survey scanners, the compound scan (top) shows the irregular shape and angular, microlobulated margins of the tumor and the structures of the breast parenchyma better than the simple scan (bottom). If the mass were interpreted as circumscribed, on a simple scan using the conventional vertical beam, the diagnosis would have been missed. It is easier, however, to appreciate the posterior features, both the enhancing and attenuating effects of the tumor on sound transmission (b). b Invasive ductal carcinoma, 2 × 1.5cm. The compound scan (top) more clearly demonstrates the parenchymal anatomy of the dense breast and the combination of smooth and irregular tumor margins. Fewer anatomic structures are visible in the simple scan (bottom), but the acoustic phenomena are better appreciated. Among the diagnostic criteria for interpretation, posterior features hold less weight than shape and margins. c Invasive breast carcinoma 7 mm in diameter (arrowhead). The compound scan (top) demonstrates the parenchymal structure of the breast and the irregular tumor. The simple scan reveals a tumor shadow but also shows shadowing in the adjacent breast parenchyma. The tumor is poorly delineated. d This compound scan shows a solid, smooth, homogeneous mass whose margins can be clearly evaluated. The good sound transmission through the tumor and adjacent parenchyma are apparent in the simple scan. Thus, each scan complements the other in evaluating the mass. Using high resolution handheld transducers with spatial compounding, the posterior feature characteristics are less conspicuous but remain identifiable, sometimes with a conical appearance the apex of the cone at the posterior aspect of the scan. Fig. 2.7 a–d Effect of scanning direction on the acoustic shadow cast by an invasive breast carcinoma. a Compound scan shows a 2.5 × 3.5cm mass with irregular margins distorting the breast architecture. b Scanning from the left side with transducer 1 (left) shows fewer structural details. A heavy acoustic shadow projects to the right from the mass. c Scanning from the right front with transducer 3 demonstrates an acoustic shadow directed posteriorly and to the left. d Scanning from the right side with transducer 4 demonstrates a shadow directed to the left. It is apparent from all images that the shadow emanates from the mass and is not caused by breast parenchyma. Fig. 2.8 a, b Compound scans: comparison of both breasts with and without compression in a patient with left breast carcinoma. a Scan of the right breast without compression shows marked central sound absorption (top). Compression eliminates the hypoechoic region to depict the subareolar breast architecture (bottom). b Left breast scan with minimal compression also shows prominent central attenuation. It is unclear whether the shadow is cast by a tumor or is an artifact caused by breast parenchyma. The compression scan (bottom) reveals a deeply situated, hypoechoic focal lesion with irregular margins, consistent with a large invasive carcinoma. It is also important that the focal zones should be set at the area of interest. Fig. 2.9 a, b Noncompression and compression compound scans of breast carcinoma. a Without compression a hypoechoic mass 2 × 1.5cm can be seen (top). Compression of the breast does not alter the shape of the mass (bottom) identifiable now as a tumor. An artifact or fatty infiltration would have changed in response to tissue compression. b Noncompression compound scan (top) shows a mass with irregular margins 2.5 × 1cm in size (arrowhead). Internal echoes make the mass difficult to detect, but there is obvious local architectural distortion. Compression deforms the breast parenchyma, making the anatomic structures difficult to interpret (bottom). The mass, whose echogenicity is similar to that of the parenchymal tissue, is virtually undetectable. In real-time sonographic examination, only a small segment of the breast is visualized. In general, using a recommended high-frequency broad bandwidth linear transducer the field of view is approximately 5 cm2 with a slice thickness of less than 1 mm. The small field of view is one reason that many examiners have not extended their sonographic studies beyond the focused ultrasound investigation of palpable or mammographically suspected masses (“lumpography”). Unlike water-path scanning where pressure exerted on tissue is constant, in real-time scanning the sonographer or sonologist applies variable pressure to the breast parenchyma with the ultrasound probe, and this pressure can alter the appearance of the tissue (Fig. 2.10 a–c). Thus, in real-time scanning, the examination technique should be adapted for each patient in order to optimize visualization of a specific lesion or area of interest. Focused or targeted scanning is here considered as the basic level of breast ultrasound. Its goal is to confirm a mass or other abnormality and to characterize it. Detection of nonpalpable lesions, or screening, is excluded from this type of scanning. Nevertheless, ultrasound depiction of a mass requires skill and an understanding of how to obtain a diagnostic ultrasound image. For the medial breast, the patient should be positioned supine with the hands clasped behind the neck (Fig. 2.11 a–d). This immobilizes the breast and makes the findings more reproducible. The flattening of the breast also improves sound penetration. The supine position causes the breast to flatten on the chest wall, and elevating the arms places tension on the pectoralis muscles, further helping to flatten and immobilize the breast. This facilitates a complete, systematic examination and enhances the reproducibility of findings. The same position is used in breast surgery, making it easier to identify and excise lesions that have been localized by breast ultrasound.
Water-Path Scanning
Water-Bag Technique
Immersion Technique
Real-Time Examination
Patient Positioning
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree