|
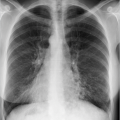
The expiratory radiograph does not permit assessment of the cardiopulmonary status since the lung is inadequately ventilated and the pulmonary vessels appear dilated. This can obscure other relevant findings, e.g., small pulmonary infiltrates or incipient congestive heart failure.
Comparability with previous or subsequent radiographs is not possible.
With modern digital equipment technology, a pneumothorax of clinically relevant size can also be recognized on an inspiratory radiograph.
obtained. The mobile detector is positioned beneath the thorax of the supine patient and the tube of the mobile radiography unit is placed above the patient. The focus detector distance should be 90 to 120 cm. For several reasons, supine radiographs have poorer image quality than standing or sitting radiographs:
Table 1.2 Quality requirements for chest radiographs5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
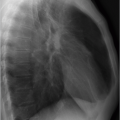
 Fig. 1.4 Grid artifact. Radiograph (supine radiograph). Different radiolucency of both axillae (arrows) as distinguishing feature of that artifact. |
The reduced focus detector distance results in greater geometric distortion; the mediastinal width and heart size appear enlarged on the supine radiograph (▶Fig. 1.2); the heart is farther away from the detector, showing greater geometric enlargement.
The diaphragm is higher, resulting in reduced inspiration depth.
Lung perfusion has no gravity-mediated caudocranial gradient; it is not possible to diagnose pulmonary blood flow redistribution.
Since the tube voltage used is lower, bone superimposition is more pronounced.
The lower generator power of mobile radiography units results in a longer exposure time and is likely to cause motion blur due to breathing or heart pulsations.
Radiation exposure: Tube voltage, tube current, and pitch should be adjusted such that the radiation exposure complies with the reference values specified for diagnostic imaging of patients of normal weight. Relevant reference values vary greatly among different countries.7
Tube voltage: For most applications, a tube voltage of 110 to 120 kVp is suitable. For computed tomography angiography (CTA), the tube voltage may be reduced in certain circumstances to 80 to 100 kVp, in particular for pediatric or slim patients.8,9
Automatic tube current modulation: Due to the major differences in the absorption profiles of the thorax in the craniocaudal and axial directions, the use of automatic tube current modulation has greatly contributed to dose reduction.8 However, there is a risk of this automated facility preselecting a very high tube current for obese patients. It is therefore recommended to limit the maximum tube current in the scan parameters if this is technically possible. Other considerations apply for low-dose CT.
Slice thickness: The detector configuration should provide for a reconstructed slice thickness of 1 to 1.5 mm. But that does not apply to CT scanners with a limited row count, for which a compromise has to be made between the minimum slice thickness possible and the scan duration. A limiting factor for the slice thickness in such cases is the maximum length of breath suspension that can be maintained before breathing artifacts degrade the image quality. There does not appear to be much benefit in selecting a slice thickness of substantially less than 1 mm in the thoracic region because of the ensuing rise in image noise; a reduced slice thickness is unlikely to confer any additional diagnostic insights of relevance.
Image reconstructions recommended for routine examinations:
5 mm axial for quick orientation also for the referring physician (soft-tissue and lung kernel).
Axial thin-slice reconstructions (1.5-3 mm) with soft-tissue kernel, in CTA possibly reduced slice thickness.
Axial thin-slice reconstructions (1-1.5 mm) with lung kernel to allow for volumetric measurements.
3-5 mm coronal and sagittal.
Overlapping of thin-slice reconstructions: To achieve a good image quality for 3D (three-dimensional) reformatting of image data and precise volumetry, overlapping reconstruction of the thin-slice series by at least 20% of slice thickness is recommended.
IV contrast: If IV contrast administration is indicated, a fixed delay of 40 s may be used for most diagnostic purposes. Alternatively, a bolus tracking procedure could be employed. Here the arrival of the contrast bolus in the descending aorta triggers the scan. An additional delay of a few seconds is advisable, for example, to accentuate the contrast between a tumor and its surrounding tissues. The use of CTA for diagnostic exploration of pulmonary embolisms requires bolus tracking or a test bolus in the pulmonary trunk or right ventricle.
Scanning direction: Examination is performed in deep inspiration. A caudocranial scanning direction helps to reduce breathing artifacts. First, the basal lung regions most susceptible to breathing artifacts are scanned, followed by the less susceptible apical regions. Furthermore, with appropriate contrast medium timing, beam hardening artifacts caused by highly concentrated contrast material in the superior vena cava and brachiocephalic veins can be reduced.
pulmonary lobule, which is not possible with a 10-mm slice thickness. The key driver of HRCT was thus to generate thin slices of the lung parenchyma (slice thickness: around 1 mm) to improve such assignment. However, sequential 1-mm slices were not suitable for continuous imaging of the entire lung at that time. The only remedy here was therefore to acquisition discontiguous slices at greater distances apart (e.g., 10 mm). This inevitably results in incomplete visualization of the lung. For diagnosis of diffuse lung diseases, a number of representative slices suffice; however, thanks to the higher spatial resolution, it has been possible to achieve a diagnostic gain but there was a risk of focal changes being overlooked.
Reduced slice thickness (maximum 1.5 mm) at greater distances apart (e.g., 10 mm).
High radiation dose for the single slice (high tube voltage and high tube current).
Edge-enhancing reconstruction kernel for maximum spatial resolution in the slice plane.
Maximum image matrix (at least 512 × 512 pixels).
absorption. Both present a risk of unnecessarily high radiation exposure or inadequate image quality when the tube current is too low. A more robust approach entails the use of a weightadapted, fixed tube current. Several lung cancer screening trials used a variety of low-dose CT protocols, which generally achieved an effective dose of approximately 1.5 mSv.16,17 These provide for a dose saving of over 80% compared with standard CT; with ultralow-dose protocols, radiation exposure similar to a chest radiograph in two views can even be achieved.15
Sequential expiratory scans: A few sequential expiratory scans, in addition to the inspiratory spiral scan, yield just slightly higher radiation exposure. Even severely dyspneic patients generally tolerate the very short breath-hold times for sequential expiratory scans. One drawback is the sampling error since only a small part of the lung parenchyma is displayed. Besides, interpretation of the findings with regard to air trapping may be difficult at times.
Expiratory volume acquisition: In addition to an inspiratory spiral CT scan, a second expiratory spiral scan is obtained across the entire thorax. Its advantage is that it displays the whole of the lung and focal air trapping is not overlooked. But this involves higher radiation exposure, although the expiratory scan can be obtained in low-dose technique. Besides, patients cannot hold their breath in expiration for as long as in inspiration. If a very fast CT scanner is not available, a compromise must be reached between slice thickness and scan duration since otherwise breathing artifacts would adversely affect image interpretation.
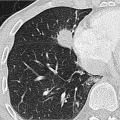
 Fig. 1.6 Expiratory CT for visualization of small airway disease. (a) Hardly any abnormalities in inspiration. (b) In expiration greater evidence of air trapping (darker areas) in the diseased lung parenchyma. |
the threshold value to start the scan high enough so that it is never reached. To begin with, the patient takes a few normal breaths under bolus tracking, followed by a few forced breaths. Eventually, the bolus tracking mode must be stopped manually. An imaging frequency of one image per second suffices for interpretation of the findings.
 Fig. 1.7 Dynamic CT of the ventilation cycle. Visualization of lung density during several breathing cycles. Illustrated in each case are measurements in the right (curve 1) and in the left lung (curve 2). (a) Normal results for the left lung (2). Density changes in one breathing cycle of more than 50 HU (Hounsfield units). On the right (1), mild air trapping with lower density amplitude. (b) Extensive air trapping. Only minor changes in lung parenchymal density; in the left lung (2) more massive air trapping than on the right (1).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|