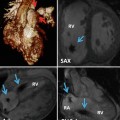
Fig. 30.1
Color-coded CT angiography of chest shows the left main bronchus (LB) surrounded by the left pulmonary veins, branches of the left pulmonary artery (LPA), and the left atrium (LA). The LPA starts superior and immediately turns posterior to the left bronchus, and in the left lower lobe mainly remains posteriorly. The left superior pulmonary vein (LSPV) courses anterior to the left bronchus. At left paramedian level (sagittal 1), the left bronchus can easily be squeezed by enlarged vessels causing left lung or left lower lobe atelectasis. An enlarged LA can increase the bronchial compression by elevating the left pulmonary veins. Sagittal 2 image is obtained through left lower lobe branches. DA descending aorta, LIPV left inferior pulmonary vein
Plastic Bronchitis
In this rare, potentially life-threatening condition patients present with recurrent expectoration of branching, mucoid airway casts, which vary in size. It can be found in children with underlying cardiac defects particularly post-shunt procedures with Fontan being the most common [7, 8]. Bronchial casts are acellular noninflammatory composed primarily of mucin and fibrin as compared to the eosinophilic inflammatory casts seen in the other conditions. The pathogenesis of plastic bronchitis includes hypersecretion of airway mucus, abnormalities of pulmonary lymphatic drainage, and poor cardiac output [7, 9]. CT can demonstrate bronchial casts causing partial or complete obstruction of the central airways with associated atelectasis and consolidation. Bronchiectasis and obstructive hyperinflation are usually absent [10]. The airway and lung abnormalities rapidly improve after removal of the bronchial casts.
Parenchymal Changes
Pulmonary edema is a complication of many types of primary cardiovascular diseases [7]. Obstruction of pulmonary venous drainage increases capillary pressure and results in transudation of fluid. Disease states that lead to an increased right atrial pressure impair drainage of pulmonary lymphatics resulting in fluid retention and, often, pleural effusions (right more than left). Left-to-right shunts increase pulmonary blood flow leading to water retention in the lungs.
Pulmonary emphysematous changes in CHD are usually led by airway obstruction. In a recent study by Nabo et al., the frequency of pulmonary emphysema in CHD patients with increased pulmonary blood flow due to left-to-right shunting was assessed using MDCT [11]. The emphysematous change was detected in 44 % of patients. The frequency of segmental emphysematous change in left side was higher than in right side (14.8 % vs. 6.5 %). The left main bronchus is surrounded by the left pulmonary artery, left pulmonary veins, and left atrium (Fig. 30.1). These anatomical features make the left lung vulnerable to develop lobar emphysema. Lobar emphysema due to extrinsic compression by vascular structures is often relieved by correction of the underlying cardiac or vascular defect [12].
Palliative univentricular physiology (so-called Fontan physiology) is associated with passive, non-pulsatile drainage to the pulmonary arteries. Patients typically have congested, small lungs with a restrictive pattern of lung function. The reason why lungs are smaller is unclear but may be related to previous pleurodesis in some cases [7]. Alternatively, there may be an inherent degree of pulmonary hypoplasia due to the lack of pulsatile flow to stimulate lung growth. There is also a high risk of pulmonary embolic disease in this slow-flow state.
Pectus Excavatum
Pectus excavatum is the most common congenital chest wall malformation and is usually seen as an isolated congenital abnormality. However, it is occasionally associated with cardiac abnormalities, such as Marfan’s syndrome or CHD (5–10 %) [13]. Cardiac or pulmonary compression can result is restrictive lung disease and decreased right ventricle function (Fig. 30.2). Cardiac compression can also contribute to postoperative hemodynamic instability if the pectus deformity is left uncorrected during repair of the CHD. Common CHDs in association with pectus excavatum include VSD, ASD, TOF, double outlet right ventricle, transposition of the great arteries, and aortic coarctation. Aortic aneurysm and dissection are not unusual presentation (Fig. 30.2).
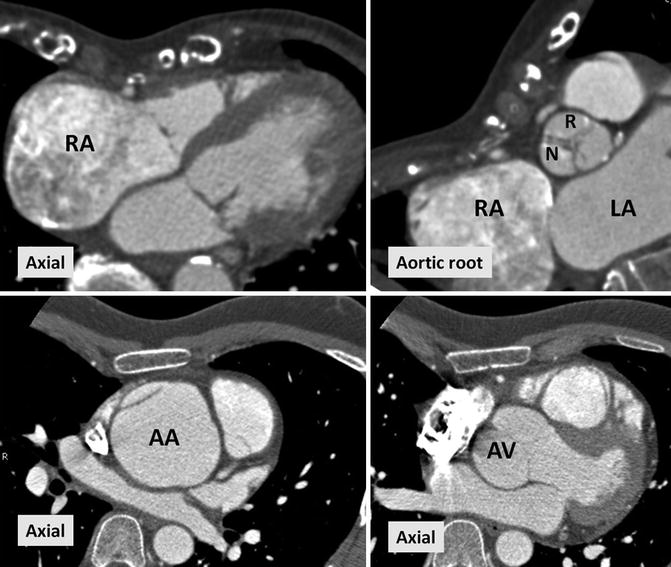

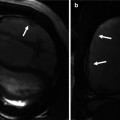
Fig. 30.2
CT images in two different patients with Marfan’s syndrome and pectus excavatum. Upper row is a 22-year-old male with severe pectus excavatum causing right ventricle outflow tract narrowing and right atrium dilatation (RA). Aortic root view shows mild counterclockwise rotation of the aortic valve. Lower row demonstrates aortic aneurysm and focal dissection in a 24-year-old male. AA ascending aorta, AV aortic valve, N noncoronary sinus, R right coronary sinus
Intrapulmonary Vascular Collaterals and Shunts
Intrapulmonary systemic–pulmonary collaterals (SPC) develop frequently with a bidirectional cavopulmonary connections (BCPC) or Fontan circulation [14]. These systemic to pulmonary shunts usually develop between bronchial and pulmonary vessels causing arterial–arterial or venous–venous communications [15]. Both MR and CT angiography can show the extent of large collaterals. However microscopic communications are difficult to see directly. The mechanism for the development of collateral vessels in univentricular cases is unknown. Post-Fontan patients typically have passive, non-pulsatile drainage to the pulmonary arteries. This slow-flow state may lead to the development of systemic to pulmonary collateral vessels. Vascular endothelial growth factor and or lack of hepatic venous effluent may also play a role [15–18]. SPC develops more in the lung with less pulmonary arterial flow. Left-to-right shunting via SPCs competes with volume-unloading effect of Fontan. This can contribute to increasing cyanosis, diastolic heart failure, and increased pulmonary artery pressure [19, 20]. Measurement of the magnitude and evaluation of hemodynamic consequences of these shunts are then important. The magnitude of SPC flow can be large and may approach 20–25 % of pulmonary venous return and 18–21 % of aortic output [21]. Evaluation of shunt can be difficult using routine CT or MR angiographic methods, but it can reliably be assessed with phase-contrast MR [19, 20]. It is possible to quantify APC blood flow by subtracting the pulmonary arterial from the pulmonary venous blood flow volume for each lung. For this purpose, Whitehead and colleagues [19] used two-dimensional phase-contrast MR velocity mapping to noninvasively quantify SPC in single-ventricle patients after superior BCPC. Using this method, multiple measurements should be performed at each pulmonary artery and vein levels. For correct quantification of the flow volumes during different phases of cardiac cycle, the phase-contrast sequence should have enough temporal resolution (i.e., 20 or more reconstructed phases per cardiac cycle). Appropriate velocity encoding (VENC) setting is required for correct venous and arterial flow analysis. Due to complex anatomy complete analysis may not be achieved in all patients. Newer 4D velocity mapping technique may be more reliable and even faster than two-dimensional velocity acquisition for quantitative analysis of systemic and pulmonary perfusion including SPC after palliation of single-ventricle physiology [21].
After palliative corrective surgery of TOF with pulmonary atresia, it is not uncommon to find enlarged bronchial arteries and chest wall collaterals (Fig. 30.3). Hemoptysis can result from rupture of these collaterals in to the airways. These enlarged bronchial arteries may be part of major aortopulmonary collaterals (MAPCAs) developing in patients with pulmonary atresia and VSD (i.e., tetralogy of Fallot with pulmonary valve atresia) [22]. Enlarged intercostobronchial arteries can be identified in 85 % and subclavian artery chest wall collaterals are seen in 50 % of MAPCAs [23]. For TOF with pulmonary atresia and MAPCAs, a staged surgical approach is usually used. The operative sequence begins with a central aortopulmonary shunt followed by unifocalization of aortopulmonary collateral arteries depending on the source and distribution of pulmonary blood flow. Embolization may be necessary for severe hemoptysis, and stenosis can be corrected with balloon angioplasty and stenting [24, 25]. With CT angiography the extent of bronchial collaterals, stenotic segments, and in selected cases the bleeding site can be detected.
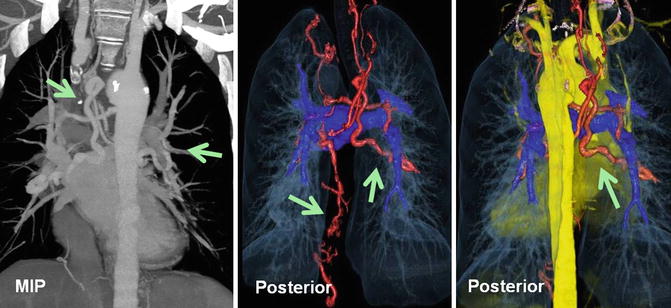

Fig. 30.3
A 35-year-old male with repaired tetralogy of Fallot presented with hemoptysis. Coronal maximum intensity pixel and color-coded multilayer CT angiography images are shown. Enlarged systemic collateral arteries (green arrows) arising from the descending thoracic aorta (bronchial), ascending cervical artery, left internal mammary artery, and celiac trunk supplying the lungs (red vessels). Pulmonary arteries are relatively small (dark blue)
Venovenous collateral vessels can develop between the two cava, the bronchial veins and the caval system, and the upper extremities veins and the pulmonary veins or the left atrium (Fig. 30.4).
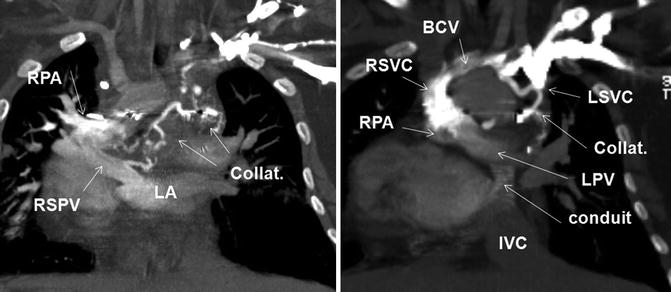

Fig. 30.4
A 17-year-old female with repaired double outlet right ventricle status post-Fontan with hemoptysis and hypoxemia and mild cyanosis. Coronal CT images after injection of contrast into left arm are shown. CT shows partial occlusion of the left brachiocephalic vein (BCV) and extensive mediastinal venous collateral (collat.) formation. There is a relatively large connection of venous collaterals with the right superior pulmonary vein (RSPV) causing early filling of the left atrium (LA) indicating a right-to-left shunt. Note the left superior vena cava (LSVC) is opacified and appears ligated. The right superior vena cava (RSVC) is connected to the right pulmonary artery (PRA). The inferior vena cava (IVC) flow is directed to the left pulmonary vein (LPV) by a conduit. Dextrocardia exists
Pulmonary Arteriovenous Malformations
Pulmonary arteriovenous malformations (PAVM), also referred to as pulmonary arteriovenous fistulas (PAVF), can occur after superior cavopulmonary anastomosis [26] (Fig. 30.5). Surgical connection of the superior vena cava (SVC) to the right pulmonary artery as classically described by Glenn was initially considered a satisfactory means of palliation for various forms of cyanotic CHD. Substantial morbidity and mortality from the late onset of ipsilateral PAVMs lead to modification of the procedure in which the classic Glenn procedure was replaced by BCPA. Although physiologically similar to the classic Glenn operation, BCPA has been shown to cause bilateral rather than ipsilateral PAVMs under certain conditions, but at a lower incidence [26, 27]. The main reason for the lower incidence may be that the BCPA is usually performed as part of a staged univentricular palliation, with most patients proceeding to incorporation of the inferior vena cava (IVC) and hepatic venous flow into the pulmonary circulation, namely, completion Fontan operation within 1–3 years. An exception to this pattern is the population of patients with polysplenia syndrome and azygos continuation of an interrupted IVC. Such patients commonly develop PAVMs after a Kawashima procedure (BCPA in the setting of azygos continuation of an interrupted IVC to the SVC) [28] (Fig. 30.5). In this procedure most of the systemic venous (85 %) return to the pulmonary circulation, while the hepatic and coronary sinus venous return remains to the right atrium [28]. The PAVMs seen in this population are similar to the hepatopulmonary syndrome seen in children with end-stage liver disease. In other words, exclusion of hepatic blood flow from the pulmonary blood flow contributes to the development of PAVM. In cases of bilateral SVC connections to the left and right pulmonary arteries, the relatively decreased pulmonary circulation on the opposite side to the IVC flow often results in increased AVMs on that side (right side greater than left) [29].
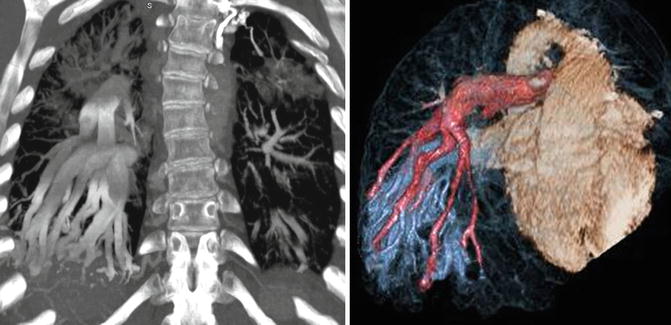

Fig. 30.5
Coronal and color-coded volume-rendered CT images of chest demonstrate extensive arteriovenous malformations (AVM) mainly in the right lower lobe in a patient with bidirectional cavopulmonary connections/Kawashima procedure presented with hypoxemia. Right AVMs did not resolve after hepatic vein inclusion into the left pulmonary circulation. Multiple coil embolizations of the AVMs had been performed before this study. Pulmonary artery branches are colored in pink
The primary consequence of PAVMs is decreased systemic oxygen saturation due to right-to-left shunting. Resolution of PAVMs after hepatic vein inclusion into the cavopulmonary circulation has been reported [30]. In this procedure, the hepatic veins are taken off of the atrium with a cuff of atrial tissue and anastomosed to the underside of the ipsilateral pulmonary artery with an extracardiac conduit (13–18 mm) or using a lateral caval tunnel constructed with a PTFE (Gore-Tex; W.L. Gore & Associates, Newark, DE) patch [30].
CT findings include the presence of abnormally enlarged pulmonary vessels extending to the periphery of the lung with the PAVM forming a small tangle of vessels (Fig. 30.5). The use of thick-slab (4–20 mm) maximum intensity projections (MIPs) and volume-rendering reconstructions can help detecting and characterizing PAVMs on CT [31].
Palliative Pulmonary Vascular Shunts
Surgical palliation for neonates and children with diminished pulmonary artery blood flow is usually accomplished with systemic–pulmonary artery shunts. TOF is the most common CHD cases who had a palliative shunt procedure in the past (70 %) [32]. In complex CHD, this procedure is performed before cavopulmonary connection. In CT or MR angiographic assessment of this group of adult CHD patients, it is not uncommon to see the remnant of the shunt after surgical closure or embolization or rarely an intact systemic–pulmonary artery shunt (Fig. 30.6). In some cases with history of modified or classic Blalock–Taussig shunts distortion and stenosis (up to 20 %) of the pulmonary artery at the site of the shunt anastomosis can be seen [33, 34].
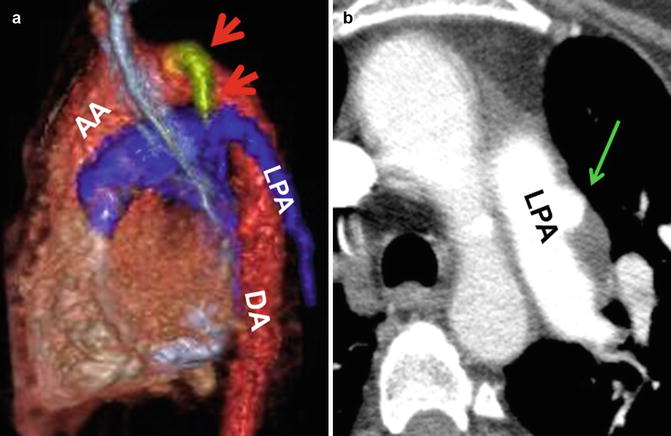

Fig. 30.6
Extracardiac palliative systemic–pulmonary shunts. (a) Classic Blalock–Taussig anastomotic shunt is seen between the left subclavian artery (red arrows) and left pulmonary artery (LPA) in a patient with repaired tetralogy of Fallot. (b) Left pulmonary artery (LPA) partially thrombosed aneurysm (green arrow) possibly at the site of a closed shunt in a patient with repaired tetralogy. AA ascending aorta, DA descending aorta
Pulmonary Hypertension
Pulmonary hypertension is defined as mean pulmonary artery pressure greater than 25 mmHg at rest, or as pulmonary vascular resistance greater than 3 Wood units. According to the current WHO classification [35], pulmonary hypertension is divided into five classes: (1) pulmonary arterial hypertension (idiopathic, veno-occlusive, etc.), (2) secondary to left heart diseases, (3) associated with lung diseases and/or hypoxemia, (4) secondary to chronic thrombotic and/or embolic disease, and (5) miscellaneous. Pulmonary hypertension associated with CHD is commonly assigned to the first category [36]. The likely mechanism behind the development of pulmonary hypertension in CHD, as in idiopathic pulmonary hypertension, is multifactorial. Vasoconstriction, proliferative and obstructive remodeling of the pulmonary vascular bed, inflammation, and thrombosis all appear to be involved [35, 37]. If pulmonary vascular resistance increases to the point where pulmonary artery pressure exceeds systemic blood pressure, right ventricular diastolic and systolic function decrease acutely and can rapidly progress to right ventricular dilation, dysfunction, and failure [38, 39]. Among Eisenmenger patients, the response of the right ventricle tends to resemble idiopathic pulmonary hypertension in those with pretricuspid defects (ASD) causing atrial and right ventricle dilatation [39]. However, in Eisenmenger patients secondary to a large VSD, the right ventricle is able to sustain an elevated afterload over a much longer period of time and becomes hypertrophied [38]. The rate of progression of pulmonary arterial disease in patients with large VSDs and PDAs is generally faster. A large ASD may also induce an increase in pulmonary arterial pressure but at much slower rate and only rarely result in severe pulmonary hypertension early in life (Fig. 30.7). Eisenmenger syndrome demonstrates pulmonary vascular changes on CT which is similar to idiopathic pulmonary hypertension. These changes include centrilobular ground-glass opacities, mosaicism, and neovascularity [40]. Neovascularity due to enlarged bronchial arteries and intercostal systemic collaterals are more common in patients with Eisenmenger syndrome especially in posttricuspid communications [41] (Fig. 30.8). However, there is no correlation between these findings and severity of pulmonary hypertension [40]. Mosaic attenuation due to pulmonary hypertension can be optimally seen on minimum-intensity projection reconstructions CT angiographies (Fig. 30.9) [42]. In the presence of severe pulmonary hypertension, intrapulmonary hemorrhage can be life-threatening. In mild cases, pulmonary hemorrhage may resolve in a few days.
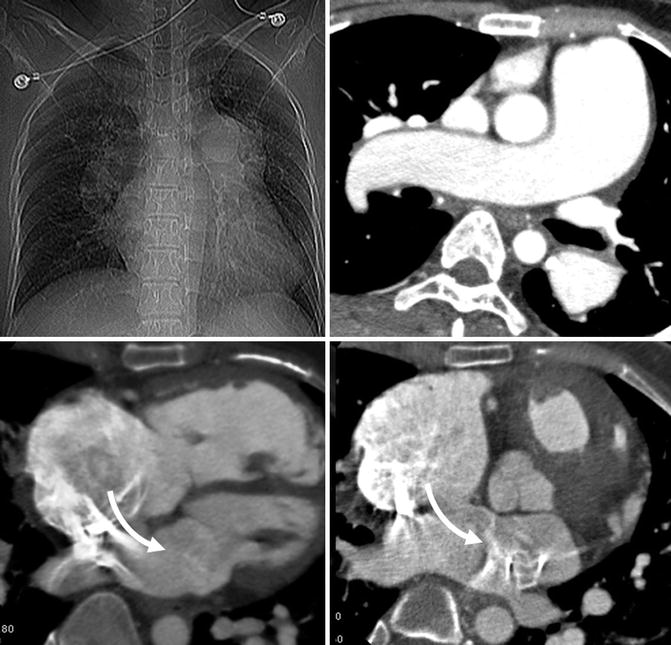
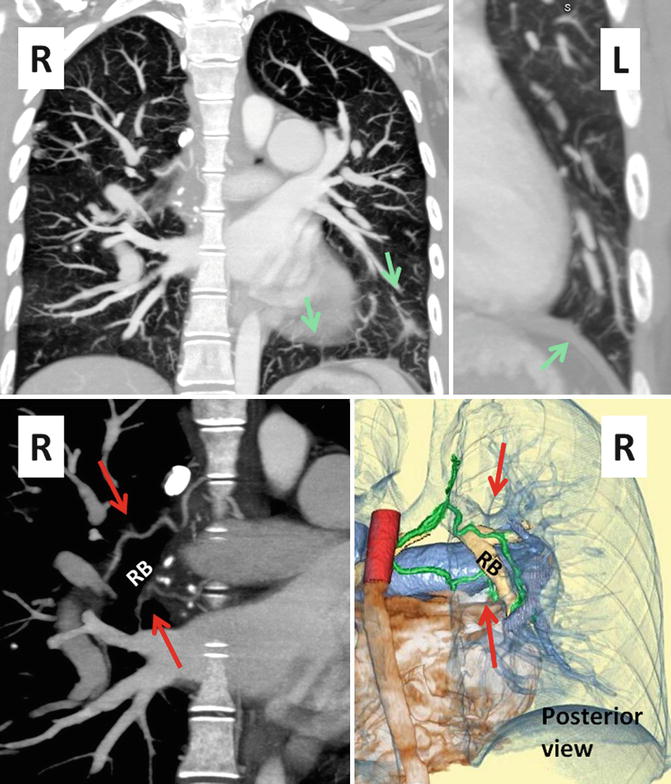
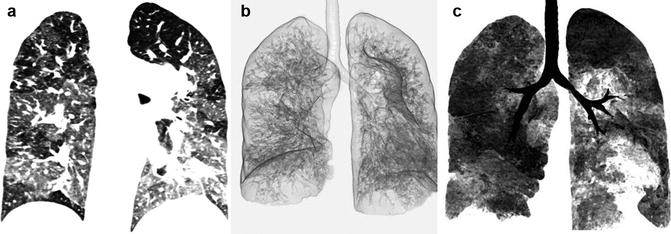

Fig. 30.7
Typical findings of Eisenmenger syndrome in a patient with large atrial septal defect (arrows). Enlarged pulmonary arteries, dilated hypertrophic right ventricle, and dilated right atrium are evident. The left atrium is mildly dilated and left ventricle is normal in size

Fig. 30.8
Coronal CT images show systemic to pulmonary collaterals (SPC) in Eisenmenger syndrome. These collaterals develop between bronchial and/or chest wall intercostal arteries on the systemic side and the intraparenchymal pulmonary arteries (green arrows). Upper row images show multiple tortuous small collateral arteries best shown in the left lower lung extending to the chest wall (green arrows). Lower row images show enlarged bronchial arteries (red arrows) along the right bronchus (RB)

Fig. 30.9
Mosaic attenuation of the lungs in Eisenmenger syndrome. (a) Maximum intensity pixel. (b) Three-dimensional minimum-intensity pixel. (c) Coronal thin slab minimum-intensity pixel. Note improved demonstration of the abnormal perfusion with minimum-intensity pixel technique
Thromboembolic Complications
Eisenmenger syndrome typically results in severe dilation of the pulmonary arteries, sluggish flow, and mural thrombus formation of the central pulmonary arteries affecting 20–30 % of patients [43, 44]. Pulmonary artery calcifications are seen more frequently in patients with pulmonary thrombosis. Women and patients with lower oxygen saturations are at the highest risk of developing thrombosis [44]. Thrombosis of the central pulmonary arteries may be the source for peripheral pulmonary thrombi and pulmonary infarction leading to hemoptysis. CT angiography of chest can provide information about the size of pulmonary arteries and the presence of in situ thrombosis, as well as right ventricular dimensions and function and associated cardiac or extracardiac lesions. It is shown by MRI that pulmonary arterial thrombosis among adults with Eisenmenger syndrome relates to older age, biventricular dysfunction, and slow pulmonary artery blood flow rather than degree of cyanosis or coagulation abnormalities [45].
Thrombosis of the right-sided circulation and chronic pulmonary embolic disease are complications of single-ventricle palliative procedures with an incidence of 5–16 % [46] (Figs. 30.10 and 30.11). The increased risk of thromboembolic complications in this patient population is thought to be multifactorial. Ligation of the pulmonary trunk results in a blind cul-de-sac distal to the pulmonary valve where thrombi can form. The pulmonary blood flow after these procedures is passive and dependent on the transpulmonary gradient between the pulmonary artery and left atrium. The classic Fontan circuit is associated with right atrial dilatation and slow flow, increasing the risk of atrial thrombus [47] (Fig. 30.10). Though the lateral tunnel and extracardiac conduit Fontan modifications result in better streaming of blood flow between the IVC and pulmonary artery, the risk of thromboembolism is still high [48, 49].
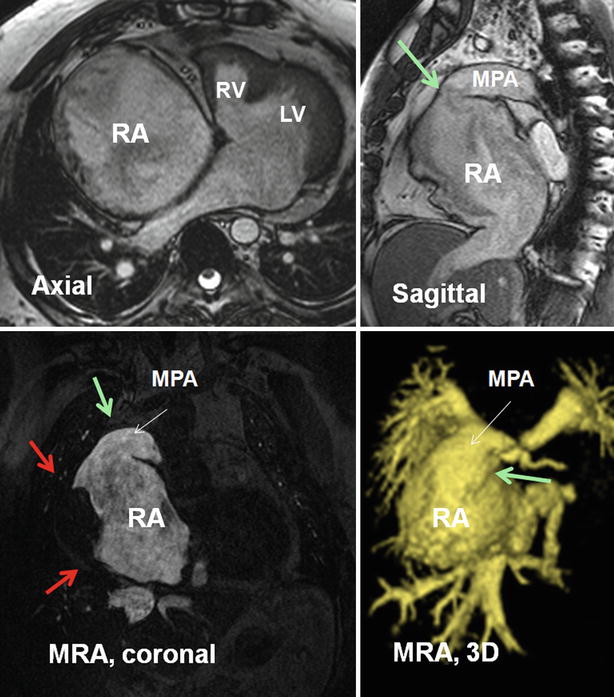
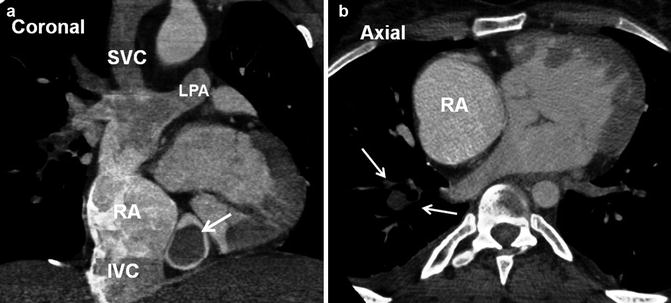

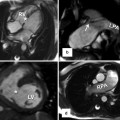
Fig. 30.10
MR images show a large right atrial mural thrombus (red arrows) in a 50-year-old male with tricuspid atresia, status post classic atriopulmonary Fontan. The anastomosis between the right atrium (RA) and main pulmonary artery (MPA) is shown by green arrows. The right atrium (RA) is massively enlarged. A large mural thrombus is shown along the lateral wall of right atrium (red arrows). Pulmonary emboli were confirmed on chest CT angiography (not shown). The right ventricle is hypoplastic and a large ventricular septal defect is seen. The tricuspid valve is absent. The MPA was disarticulated distal to the pulmonary valve (not shown). The blind pouch of MPA could also be a hidden location for thrombus formation. It was clear in this patient. Coronal and three-dimensional (3D) images of a right heart MR angiography (MRA) are presented. LV left ventricle, RV right ventricle

Fig. 30.11
A 22-year-old male with tricuspid atresia, status post bidirectional cavopulmonary connection (BCPC) and intracardiac Fontan presented with shortness of breath. CT angiography showed multiple pulmonary emboli (arrows in b). A large thrombus is shown in a dilated coronary sinus (arrow in a). The right atrium (RA) is moderately dilated. IVC inferior vena cava, LPA left pulmonary artery, SVC superior vena cava
Infected systemic to pulmonary shunts (aortopulmonary and ventriculopulmonary) may produce septic pulmonary emboli [50]. The development of right-to-left shunts can lead to systemic embolism from a clot in the systemic vein.
When evaluating for intravascular thrombosis, first-pass imaging with CT or MR angiography is the ideal method to evaluate for intravascular filling defects. However, a high prevalence of variant vascular anatomy in this patient population, such as bilateral SVCs or interrupted IVC with polysplenia, can cause mixing of contrast and unopacified blood resulting in pseudo-filling defects that can limit evaluation for intravascular thrombus (Fig. 30.12). To keep mixing artifacts at a minimum, the site of intravenous contrast injection should be carefully selected based on a patient’s known vascular anatomy. Simultaneous injection of contrast agent from more than one site, e.g., simultaneous injection of contrast agent from both an upper and a lower extremity vein to adequately opacify the Glenn and Fontan circuits, can help avoid pitfalls due to mixing artifacts when performing first-pass imaging [51]. Dual-phase imaging, with a second set of images in the equilibrium phase, can also help circumvent this problem. While this is done routinely with MR angiography, a case-by-case decision has to be made with CT angiography to keep the radiation exposure as low as reasonably possible.
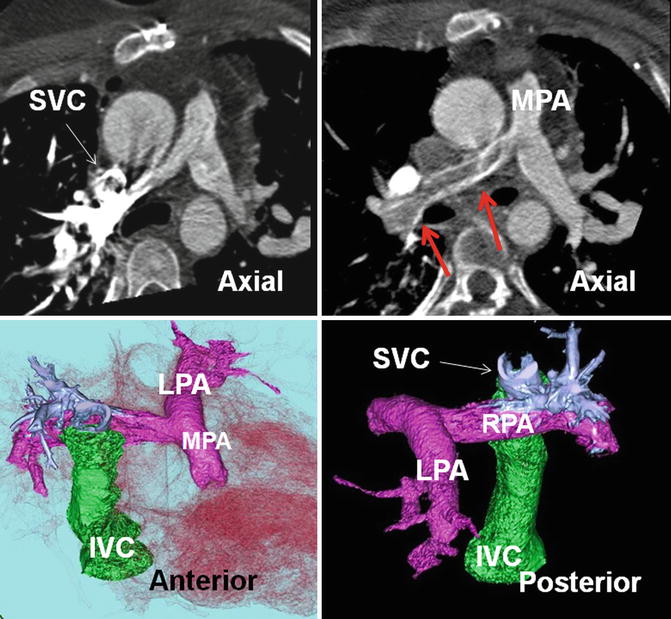

Fig. 30.12
Pseudothrombus of right pulmonary artery (red arrows) due to streaming of nonenhanced inferior vena cava (IVC) flow. In a patient with bidirectional Glenn and extracardiac Fontan (green structure) for pulmonary atresia. Axial CT scans and color-coded images are presented. Injection of contrast is performed in the right arm causing severe enhancement of the superior aspect of right pulmonary artery (shown in blue in color images). The inferior margin remained nonopacified (red arrows) due to streaming of noncontrast blood of the IVC entering the right pulmonary artery (RPA) by extracardiac Fontan tunnel (green structure). To avoid this problem simultaneous contrast injection into the upper and lower extremity veins may be necessary. LPA left pulmonary artery, MPA main pulmonary artery, SVC superior vena cava
Paradoxical Embolism
The risk of paradoxical embolism in CHD is high and can be related to intracardiac or extracardiac shunts. Paradoxical embolism can be associated with cerebral infarct, brain abscess, fat embolism after trauma, and limb ischemia [52, 53]. In this regard CT and MR have crucial role in diagnosis of the primary cause and secondary complications of paradoxical embolism. Direct assessment of an intracardiac shunt is mainly performed by echocardiography and MRI; for extracardiac shunts, CT or MR is commonly performed. However, given the widespread use of cardiac CT for other indications, it has recently gained momentum for the analysis of intracardiac shunts. An indirect diagnosis of an intracardiac shunt can also be performed by transcranial Doppler sonography.
Similar to patients with patent foramen ovale (PFO), non-cyanotic intracardiac shunts including ASD, VSD, and PDA, along with precipitating hemodynamics, can result in paradoxical embolism [52]. In a retrospective study, Bannan et al. demonstrated that 14 % of patients undergoing ASD closure for an indication of significant left-to-right shunt presented with paradoxical embolism [52]. This incidence may be higher than expected, and a diagnosis of paradoxical embolism should be considered more frequently in patients with ASD and cryptogenic stroke [52]. Cyanotic CHDs typically encompass multiple or severe structural abnormalities that allow large volumes of venous blood to shunt directly into the systemic circulation. Paradoxical embolism can complicate the problems in patients with TOF, Eisenmenger syndrome, transposition of the great arteries, and truncus arteriosus [54–57].
In repaired CHD, the presence of residual intracardiac shunts can increase the risk of paradoxical embolism. Patients with transposition of the great arteries and concomitant baffle leaks may have an increased risk of paradoxical embolism [58] (Fig. 30.13). It is important to exclude baffle leak if the patient is scheduled for intracardiac lead placement [59, 60]. A potential site of interatrial communication may be seen by cardiac CT through enlarged Thebesian veins passing along superior interatrial muscle bundle (Bachmann) between the right and left atrial appendages (Fig. 30.14).
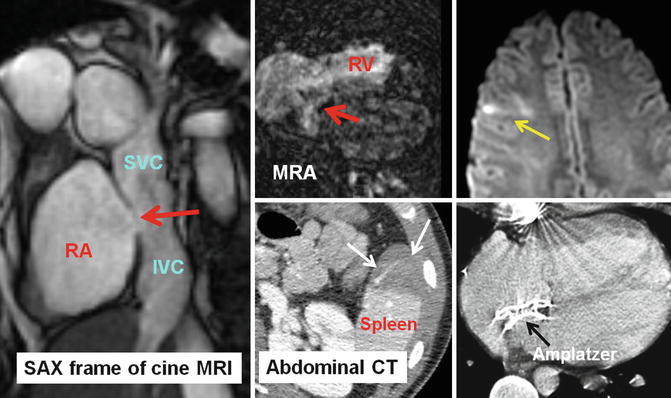





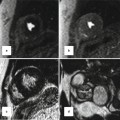
Fig. 30.13
A 24-year-old male with transposition of great arteries status post-Mustard operation evaluated for the cause of brain infarction. MRI of brain showed embolic infarct in the distribution of middle cerebral artery (yellow arrow) and CT scan of the abdomen showed anterior splenic infarction (white arrows). A large baffle defect was shown by MRI (red arrows) which subsequently closed with a transcatheter Amplatzer device (shown in CT of chest). IVC inferior vena cava, MRA magnetic resonance angiography, RA right atrium, RV right ventricle, SAX short axis, SVC superior vena cava
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree