Fleischner Society Glossary of Terms for Thoracic Imaging
25.1 Preliminary Remarks
The Fleischner Society is an international society for thoracic radiology, founded in 1969 to provide a forum for the presentation and discussion of scientific matters.1 It has for decades been active in defining and standardizing terms to describe findings in thoracic imaging. As a consequence, the Society published the first glossary of terms for thoracic imaging in 1984.2 Updates to that glossary were issued in 19963 and 2008.4 The glossary presented here is the 2008 version and newest one available. Since then, the Fleischner Society has published further recommendations on specific topics—in particular, on subsolid pulmonary nodules5 and emphysema.6 Relevant terms not contained in the 2008 glossary but defined in these more recent publications have been incorporated in this chapter in a separate section (Section 25.3). Moreover, a few terms not contained in the glossary, but thought to be useful for understanding the definitions, have been added to that section (e.g., mucoid impaction and random distribution).
25.2 Glossarya
Acinus
Anatomy—The acinus is a structural unit of the lung distal to a terminal bronchiole and is supplied by first-order respiratory bronchioles; it contains alveolar ducts and alveoli. It is the largest unit in which all airways participate in gas exchange and is approximately 6 to 10 mm in diameter. One secondary pulmonary lobule contains between 3 and 25 acini.7
Radiographs and CT scans—Individual normal acini are not visible, but acinar arteries can occasionally be identified on thin-section CT scans. Accumulation of pathologic material in acini may be seen as poorly defined nodular opacities on chest radiographs and thin-section CT images. (See also nodules.)
 Fig. 25.1 Transverse CT scan in a patient with acute interstitial pneumonia. |
Acute interstitial pneumonia, or AIP
Pathology—The term acute interstitial pneumonia is reserved for diffuse alveolar damage of unknown cause. The acute phase is characterized by edema and hyaline membrane formation. The later phase is characterized by airspace and/or interstitial organization.8 The histologic pattern is indistinguishable from that of acute respiratory distress syndrome.
Radiographs and CT scans—In the acute phase, patchy bilateral ground-glass opacities are seen,9 often with some sparing of individual lobules, producing a geographic appearance; dense opacification is seen in the dependent lung (▶Fig. 25.1). In the organizing phase, architectural distortion, traction bronchiectasis, cysts, and reticular opacities are seen.10
 Fig. 25.2 Transverse CT scan shows air bronchogram as air-filled bronchi (arrows) against background of high-attenuation lung. |
Air bronchogram
Radiographs and CT scans—An air bronchogram is a pattern of air-filled (low-attenuation) bronchi on a background of opaque (high-attenuation) airless lung (▶Fig. 25.2). The sign implies (1) patency of proximal airways and (2) evacuation of alveolar air by means of absorption (atelectasis) or replacement (e.g., pneumonia) or a combination of these processes. In rare cases, the displacement of air is the result of marked interstitial expansion (e.g., lymphoma).11
Air crescent
Radiographs and CT scans—An air crescent is a collection of air in a crescentic shape that separates the wall of a cavity from an inner mass (▶Fig. 25.3). The air crescent sign is often considered characteristic of either Aspergillus colonization of preexisting cavities or retraction of infarcted lung in angioinvasive aspergillosis.12,13 However, the air crescent sign has also been reported in other conditions, including tuberculosis, Wegener granulomatosis, intracavitary hemorrhage, and lung cancer. (See also mycetoma.)
 Fig. 25.4 Transverse CT scans at end expiration shows air trapping. |
Air trapping
Pathophysiology—Air trapping is retention of air in the lung distal to an obstruction (usually partial).
CT scans—Air trapping is seen on end-expiration CT scans as parenchymal areas with less than normal increase in attenuation and lack of volume reduction. Comparison between inspiratory and expiratory CT scans can be helpful when air trapping is subtle or diffuse14,15 (▶Fig. 25.4). Differentiation from areas of decreased
attenuation resulting from hypoperfusion as a consequence of an occlusive vascular disorder (e.g., chronic thromboembolism) may be problematic,16 but other findings of airways versus vascular disease are usually present. (See also mosaic attenuation pattern.)
attenuation resulting from hypoperfusion as a consequence of an occlusive vascular disorder (e.g., chronic thromboembolism) may be problematic,16 but other findings of airways versus vascular disease are usually present. (See also mosaic attenuation pattern.)
Airspace
Anatomy—An airspace is the gas-containing part of the lung, including the respiratory bronchioles but excluding purely conducting airways, such as terminal bronchioles.
Radiographs and CT scans—This term is used in conjunction with consolidation, opacity, and nodules to designate the filling of airspaces with the products of disease.17
Aortopulmonary window
Anatomy—The aortopulmonary window is the mediastinal region bounded anteriorly by the ascending aorta, posteriorly by the descending aorta, cranially by the aortic arch, inferiorly by the left pulmonary artery, medially by the ligamentum arteriosum, and laterally by the pleura and left lung.18,19
Radiographs and CT scans—Focal concavity in the left mediastinal border below the aorta and above the left pulmonary artery can be seen on a frontal radiograph (▶Fig. 25.5). Its appearance may be modified by tortuosity of the aorta. The aortopulmonary window is a common site of lymphadenopathy in a variety of inflammatory and neoplastic diseases.
Apical cap
Pathology—An apical cap is a caplike lesion at the lung apex, usually caused by intrapulmonary and pleural fibrosis pulling down extrapleural fat20 or possibly by chronic ischemia resulting in hyaline plaque formation on the visceral pleura.21 The prevalence increases with age. It can also be seen in hematoma resulting from aortic rupture or in other fluid collection associated with infection or tumor, either outside the parietal pleura or loculated within the pleural space.22
Radiographs and CT scans—The usual appearance is of homogeneous soft-tissue attenuation capping the extreme lung apex (uni- or bilaterally), with a sharp or irregular lower border (▶Fig. 25.6). Thickness is variable, ranging up to about 30 mm.20 An apical cap occasionally mimics apical consolidation on transverse CT scans.
Architectural distortion
Pathology—Architectural distortion is characterized by abnormal displacement of bronchi, vessels, fissures, or septa caused by diffuse or localized lung disease, particularly interstitial fibrosis.
CT scans—Lung anatomy has a distorted appearance and is usually associated with pulmonary fibrosis (▶Fig. 25.7) and accompanied by volume loss.
 Fig. 25.8 Transverse CT scan shows atelectasis of right upper lobe as increased attenuation adjacent to the mediastinum. |
Atelectasis
Pathophysiology—Atelectasis is reduced inflation of all or part of the lung.23 One of the commonest mechanisms is resorption of air distal to airway obstruction (e.g., an endobronchial neoplasm).24 The synonym collapse is often used interchangeably with atelectasis, particularly when it is severe or accompanied by obvious increase in lung opacity.
Radiographs and CT scans—Reduced volume is seen, accompanied by increased opacity (chest radiograph) or attenuation (CT scan) in the affected part of the lung (▶Fig. 25.8). Atelectasis is often associated with abnormal displacement of fissures, bronchi, vessels, diaphragm, heart, or mediastinum.25 The distribution can be lobar, segmental, or subsegmental. Atelectasis is often qualified by descriptors such as linear, discoid, or platelike. (See also linear atelectasis, rounded atelectasis.)
Azygoesophageal recess
Anatomy—The azygoesophageal recess is a right posterior mediastinal recess into which the edge of the right lower lobe extends. It is limited superiorly by the azygos arch, posteriorly by the azygos vein and pleura anterior to the vertebral column, and medially by the esophagus and adjacent structures.
Radiographs and CT scans—On a frontal chest radiograph, the recess is seen as a vertically oriented interface between the right lower lobe and the adjacent mediastinum (the medial limit of the recess). Superiorly, the interface is seen as a smooth arc with convexity to the left. Disappearance or distortion of part of the interface suggests disease (e.g., subcarinal lymphadenopathy). On CT scans, the recess (▶Fig. 25.9) merits attention because small lesions located in the recess will often be invisible on chest radiographs.26
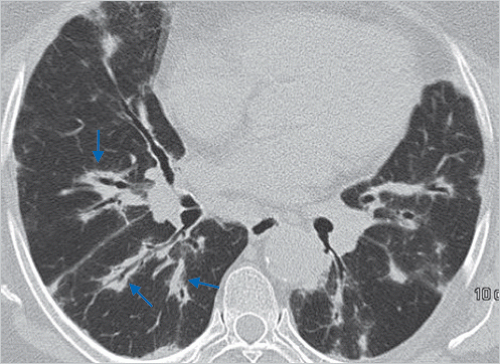
Beaded septum sign
CT scans—This sign consists of irregular and nodular thickening of interlobular septa reminiscent of a row of beads (▶Fig. 25.10). It is frequently seen in lymphangitic spread of cancer and less often in sarcoidosis.27
Bleb
Anatomy—A bleb is a small gas-containing space within the visceral pleura or in the subpleural lung, not larger than 1 cm in diameter.28
CT scans—A bleb appears as a thin-walled cystic air space contiguous with the pleura. Because the arbitrary (size)distinction between a bleb and bulla is of little clinical importance, the use of this term by radiologists is discouraged.
Bronchiectasis
Pathology—Bronchiectasis is irreversible localized or diffuse bronchial dilatation, usually resulting from chronic infection, proximal airway obstruction, or congenital bronchial abnormality.29 (See also traction bronchiectasis.)
Radiographs and CT scans—Morphologic criteria on thin-section CT scans include bronchial dilatation with respect to the accompanying pulmonary artery (signet ring sign), lack of tapering of bronchi, and identification of bronchi within 1 cm of the pleural surface30 (▶Fig. 25.11). Bronchiectasis may be classified as cylindric, varicose, or cystic, depending on the appearance of the affected bronchi. It is often accompanied by bronchial wall thickening, mucoid impaction, and small-airways abnormalities.30,31,32 (See also signet ring sign.)
Bronchiole
Anatomy—Bronchioles are non-cartilage-containing airways. Terminal bronchioles are the most distal of the purely conducting airways; they give rise to respiratory bronchioles, from which the alveoli arise and permit gas exchange. Respiratory bronchioles branch into multiple alveolar ducts.33
Radiographs and CT scans—Bronchioles are not identifiable in healthy individuals, because the bronchiolar walls are too thin.7 In inflammatory small-airways disease, however, thickened or plugged bronchioles may be seen as a nodular pattern on a chest radiograph or as a tree-in-bud pattern on CT scans.
Bronchiolectasis
Pathology—Bronchiolectasis is defined as dilatation of bronchioles. It is caused by inflammatory airways disease (potentially reversible) or, more frequently, fibrosis.
CT scans—When dilated bronchioles are filled with exudate and are thick walled, they are visible as a tree-in-bud pattern or as centrilobular nodules.34,35 In traction bronchiolectasis, the dilated bronchioles are seen as small, cystic, tubular airspaces, associated with CT findings of fibrosis (▶Fig. 25.12). (See also traction bronchiectasis and traction bronchiolectasis, tree-in-bud pattern.)
Bronchocele
Pathology—A bronchocele is bronchial dilatation due to retained secretions (mucoid impaction) usually caused by proximal obstruction, either congenital (e.g., bronchial atresia) or acquired (e.g., obstructing cancer).37
Radiographs and CT scans—A bronchocele is a tubular or branching Y or V-shaped structure that may resemble a gloved finger (▶Fig. 25.13). The CT attenuation of the mucus is generally that of soft tissue but may be modified by its composition (e.g., high-attenuation material in allergic bronchopulmonary aspergillosis). In the case of bronchial atresia, the surrounding lung may be of decreased attenuation because of reduced ventilation and, thus, perfusion.
Bronchocentric
CT scans—This descriptor is applied to disease that is conspicuously centered on macroscopic bronchovascular bundles (▶Fig. 25.14). Examples of diseases with a bronchocentric distribution include sarcoidosis,38 Kaposi sarcoma,39 and organizing pneumonia.40
 Fig. 25.15 Transverse CT scan shows a broncholith (arrow). (With kind permission of B. Rehbock, Berlin, Germany.) |
Broncholith
Pathology—A broncholith, a calcified peribronchial lymph node that erodes into an adjacent bronchus, is most often the consequence of Histoplasma or tuberculous infection.
Radiographs and CT scans—The imaging appearance is of a small calcific focus in or immediately adjacent to an airway (▶Fig. 25.15), most frequently the right middle lobe bronchus. Broncholiths are readily identified on CT scans.41 Distal obstructive changes may include atelectasis, mucoid impaction, and bronchiectasis.
Bulla
Pathology—An airspace measuring more than 1 cm—usually several centimeters—in diameter, sharply demarcated by a thin wall that is no greater than 1 mm in thickness. A bulla is usually accompanied by emphysematous changes in the adjacent lung. (See also bullous emphysema.)
Radiographs and CT scans—A bulla appears as a rounded focal lucency or area of decreased attenuation, 1 cm or more in diameter, bounded by a thin wall (▶Fig. 25.16). Multiple bullae are often present and are associated with other signs of pulmonary emphysema (centrilobular and paraseptal).
Bullous emphysema
Pathology—Bullous emphysema is bullous destruction of the lung parenchyma, usually on a background of paraseptal or panacinar emphysema. (See also emphysema, bulla.)
 Fig. 25.17 Transverse CT scan shows cavitating mass in left upper lobe. |
Cavity
Radiographs and CT scans—A cavity is a gas-filled space, seen as a lucency or low-attenuation area, within pulmonary consolidation, a mass, or a nodule (▶Fig. 25.17). In the case of cavitating consolidation, the original consolidation may resolve and leave only a thin wall. A cavity is usually produced by the expulsion or drainage of a necrotic part of the lesion via the bronchial tree. It sometimes contains a fluid level. Cavity is not a synonym for abscess.
Centrilobular
Anatomy—Centrilobular describes the region of the bronchiolovascular core of a secondary pulmonary lobule.7,42,43 This term is also used by pathologists to describe the location of lesions beyond the terminal bronchiole that center on respiratory bronchioles or even alveolar ducts.
CT scans.-A small dotlike or linear opacity in the center of a normal secondary pulmonary lobule, most obvious within 1 cm of a pleural surface, represents the intralobular artery (approximately 1 mm in diameter).44 Centrilobular abnormalities include (1) nodules, (2) a tree-in-bud pattern indicating small-airways disease, (3) increased visibility of centrilobular structures due to thickening or infiltration of the adjacent interstitium, or (4) abnormal areas of low attenuation caused by centrilobular emphysema.7 (See also lobular core structures.)
 Fig. 25.18 Transverse CT scan shows centrilobular emphysema. |
Centrilobular emphysema
Pathology—Centrilobular emphysema is characterized by destroyed centrilobular alveolar walls and enlargement of respiratory bronchioles and associated alveoli.45,46 This is the commonest form of emphysema in cigarette smokers.
CT scans—CT findings are centrilobular areas of decreased attenuation, usually without visible walls, of nonuniform distribution and predominantly located in upper lung zones47 (▶Fig. 25.18). The term centriacinar emphysema is synonymous. (See also emphysema.)
Consolidation
Pathology—Consolidation refers to an exudate or other product of disease that replaces alveolar air, rendering the lung solid (as in infective pneumonia).
Radiographs and CT scans—Consolidation appears as a homogeneous increase in pulmonary parenchymal attenuation that obscures the margins of vessels and airway walls48 (▶Fig. 25.19). An air bronchogram may be present. The attenuation characteristics of consolidated lung are only rarely helpful in differential diagnosis (e.g., decreased attenuation in lipoid pneumonia49 and increased in amiodarone toxicity50).
 Fig. 25.20 Transverse CT scan shows crazy-paving pattern. |
Crazy-paving pattern
CT scans—This pattern appears as thickened interlobular septa and intralobular lines superimposed on a background of ground-glass opacity (▶Fig. 25.20), resembling irregularly shaped paving stones. The crazy-paving pattern is often sharply demarcated from more normal lung and may have a geographic outline. It was originally reported in patients with pulmonary alveolar proteinosis51 and is also encountered in other diffuse lung diseases52 that affect both the interstitial and airspace compartments, such as lipoid pneumonia.53
Cyst
Pathology—A cyst is any round circumscribed space that is surrounded by an epithelial or fibrous wall of variable thickness.54
Radiographs and CT scans—A cyst appears as a round parenchymal lucency or low-attenuating area with a well-defined interface with normal lung. Cysts have variable wall thickness but are usually thin-walled (<2 mm) and occur without associated pulmonary emphysema (▶Fig. 25.21). Cysts in the lung usually contain air but occasionally contain fluid or solid material. The term is often used to describe enlarged thin-walled airspaces in patients with lymphangioleiomyomatosis55 or Langerhans cell histiocytosis56; thicker walled honeycomb cysts are seen in patients with end-stage fibrosis.57 (See also bleb, bulla, honeycombing, pneumatocele.)
 Fig. 25.22 Transverse CT scan in a patient with desquamative interstitial pneumonia. |
Desquamative interstitial pneumonia, or DIP
Pathology—Histologically, DIP is characterized by the widespread accumulation of an excess of macrophages in the distal airspaces. The macrophages are uniformly distributed, unlike in respiratory bronchiolitis-interstitial lung disease, in which the disease is conspicuously bronchiolocentric. Interstitial involvement is minimal. Most cases of DIP are related to cigarette smoking, but a few are idiopathic or associated with rare inborn errors of metabolism.8
Radiographs and CT scans—Ground-glass opacity is the dominant abnormality and tends to have a basal and peripheral distribution (▶Fig. 25.22). Microcystic or honeycomb changes in the area of ground-glass opacity are seen in some cases.58
Diffuse alveolar damage, or DAD
See acute interstitial pneumonia.
Emphysema
Pathology—Emphysema is characterized by permanently enlarged airspaces distal to the terminal bronchiole with destruction of alveolar walls.45,46 Absence of “obvious fibrosis” was historically regarded as an additional criterion,45 but the validity of that criterion has been questioned because some interstitial fibrosis may be present in emphysema secondary to cigarette smoking.59,60 Emphysema is usually classified in terms of the part of the acinus predominantly affected: proximal (centriacinar, more commonly termed centrilobular, emphysema), distal (paraseptal emphysema), or whole acinus (panacinar or, less commonly, panlobular emphysema).
CT scans—The CT appearance of emphysema consists of focal areas or regions of low attenuation, usually without visible walls.61 In the case of panacinar emphysema, decreased attenuation is more diffuse. (See also bullous emphysema, centrilobular emphysema, panacinar emphysema, paraseptal emphysema.)
Fissure
Anatomy—A fissure is the infolding of visceral pleura that separates one lobe or part of a lobe from another; thus, the interlobar fissures are produced by two layers of visceral pleura. Supernumerary fissures usually separate segments rather than lobes. The azygos fissure, unlike the other fissures, is formed by two layers each of visceral and parietal pleura. All fissures (apart from the azygos fissure) may be incomplete.
Radiographs and CT scans—Fissures appear as linear opacities, normally 1 mm or less in thickness, that correspond in position and extent to the anatomic fissural separation of pulmonary lobes or segments. Qualifiers include minor, major, horizontal, oblique, accessory, anomalous, azygos, and inferior accessory.
Folded lung
See rounded atelectasis.
Fungus ball
See mycetoma.
Gas trapping
See air trapping.
Ground-glass opacity
Radiographs and CT scans—On chest radiographs, groundglass opacity appears as an area of hazy increased lung opacity, usually extensive, within which margins of pulmonary vessels may be indistinct. On CT scans, it appears as hazy increased opacity of lung, with preservation of bronchial and vascular margins (▶Fig. 25.23). It is caused by partial filling of airspaces, interstitial thickening (due to fluid, cells, and/or fibrosis), partial collapse of alveoli, increased capillary blood volume, or a combination of these, the common factor being the partial displacement of air.62,63 Ground-glass opacity is less opaque than consolidation, in which bronchovascular margins are obscured. (See also consolidation.)
Halo sign
CT scans—The halo sign is a CT finding of ground-glass opacity surrounding a nodule or mass (▶Fig. 25.24). It was first described as a sign of hemorrhage around foci of invasive aspergillosis.64 The halo sign is nonspecific and may also be caused by hemorrhage associated with other types of nodules65 or by local pulmonary infiltration by neoplasm (e.g., adenocarcinoma). (See also reversed halo sign.)
Hilum
Anatomy—Hilum is a generic term that describes the indentation in the surface of an organ, where vessels and nerves connect with the organ. It is the site on the medial aspect of the lung where the vessels and bronchi enter and leave the lung.
Radiographs and CT scans—A hilum appears as a composite opacity at the root of each lung produced by bronchi, arteries, veins, lymph nodes, nerves, and other tissue. The terms hilum (singular) and hila (plural) are preferred to hilus and hili, respectively; the adjectival form is hilar.
 Fig. 25.25 Transverse CT scan shows honeycombing. |
Honeycombing
Pathology—Honeycombing represents destroyed and fibrotic lung tissue containing numerous cystic airspaces with thick fibrous walls, representing the late stage of various lung diseases, with complete loss of acinar architecture. The cysts range in size from a few millimeters to several centimeters in diameter, have variable wall thickness, and are lined by metaplastic bronchiolar epithelium.54
Radiographs and CT scans—On chest radiographs, honeycombing appears as closely approximated ring shadows, typically 3 to 10 mm in diameter with walls 1 to 3 mm in thickness, that resemble a honeycomb; the finding implies end-stage lung disease. On CT scans, the appearance is of clustered cystic air spaces, typically of comparable diameters on the order of 3 to 10 mm but occasionally as large as 2.5 cm (▶Fig. 25.25). Honeycombing is usually subpleural and is characterized by well-defined walls.57 It is a CT feature of established pulmonary fibrosis.8 Because honeycombing is often considered specific for pulmonary fibrosis and is an important criterion in the diagnosis of usual interstitial pneumonia,66 the term should be used with care, as it may directly impact patient care.
 Fig. 25.26 Transverse CT scan shows reticular opacities and honeycombing in the lower zones, typical of idiopathic pulmonary fibrosis. |
Idiopathic pulmonary fibrosis
Pathology—Idiopathic pulmonary fibrosis is a specific form of chronic fibrosing interstitial pneumonia of unknown cause and is characterized by a histologic pattern of usual interstitial pneumonia.8,67
Radiographs and CT scans—The typical imaging findings are reticular opacities and honeycombing, with a predominantly peripheral and basal distribution (▶Fig. 25.26). Ground-glass opacity, if present, is less extensive than reticular and honeycombing patterns. The typical radiologic findings68,69 are also encountered in usual interstitial pneumonia secondary to specific causes, such as asbestos-induced pulmonary fibrosis (asbestosis), and the diagnosis is usually one of exclusion. (See also usual interstitial pneumonia.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree